- Record: found
- Abstract: found
- Article: found
ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients
Read this article at
Abstract
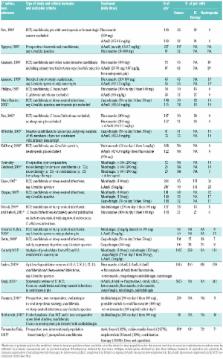
The European Conference on Infections in Leukemia (ECIL) provides recommendations for diagnostic strategies and prophylactic, pre-emptive or targeted therapy strategies for various types of infection in patients with hematologic malignancies or hematopoietic stem cell transplantation recipients. Meetings are held every two years since 2005 and evidence-based recommendations are elaborated after evaluation of the literature and discussion among specialists of nearly all European countries. In this manuscript, the ECIL group presents the 2015-update of the recommendations for the targeted treatment of invasive candidiasis, aspergillosis and mucormycosis. Current data now allow a very strong recommendation in favor of echinocandins for first-line therapy of candidemia irrespective of the underlying predisposing factors. Anidulafungin has been given the same grading as the other echinocandins for hemato-oncological patients. The beneficial role of catheter removal in candidemia is strengthened. Aspergillus guidelines now recommend the use of either voriconazole or isavuconazole for first-line treatment of invasive aspergillosis, while first-line combination antifungal therapy is not routinely recommended. As only few new data were published since the last ECIL guidelines, no major changes were made to mucormycosis recommendations.
Related collections
Most cited references65
- Record: found
- Abstract: found
- Article: not found
Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials.
- Record: found
- Abstract: found
- Article: not found
Anidulafungin versus fluconazole for invasive candidiasis.
- Record: found
- Abstract: found
- Article: not found