- Record: found
- Abstract: found
- Article: found
Induction of synapse formation by de novo neurotransmitter synthesis
Read this article at
Abstract
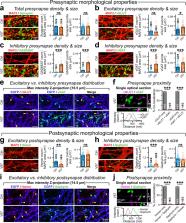
A vital question in neuroscience is how neurons align their postsynaptic structures with presynaptic release sites. Although synaptic adhesion proteins are known to contribute in this process, the role of neurotransmitters remains unclear. Here we inquire whether de novo biosynthesis and vesicular release of a noncanonical transmitter can facilitate the assembly of its corresponding postsynapses. We demonstrate that, in both stem cell-derived human neurons as well as in vivo mouse neurons of purely glutamatergic identity, ectopic expression of GABA-synthesis enzymes and vesicular transporters is sufficient to both produce GABA from ambient glutamate and transmit it from presynaptic terminals. This enables efficient accumulation and consistent activation of postsynaptic GABA A receptors, and generates fully functional GABAergic synapses that operate in parallel but independently of their glutamatergic counterparts. These findings suggest that presynaptic release of a neurotransmitter itself can signal the organization of relevant postsynaptic apparatus, which could be directly modified to reprogram the synapse identity of neurons.
Abstract
Neuronal communication relies on matching different neurostransmitter types with their appropriate receptors. The authors here demonstrate that release of a novel neurotransmitter from presynaptic terminals can induce both the accumulation and activation of its corresponding receptors on postsynaptic neurons.
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
Rapid single-step induction of functional neurons from human pluripotent stem cells.
- Record: found
- Abstract: found
- Article: not found
Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins.
- Record: found
- Abstract: found
- Article: not found