- Record: found
- Abstract: found
- Article: not found
Synthesis and structure-activity relationship studies of water-soluble β-cyclodextrin-glycyrrhetinic acid conjugates as potential anti-influenza virus agents
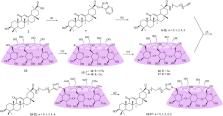
Abstract
Glycyrrhetinic acid (GA) is a major constituent of the herb Glycyrrhiza glabra, and many of its derivatives demonstrate a broad spectrum of antiviral activities. In the current study, 18 water-soluble β-cyclodextrin (CD)-GA conjugates, in which GA was covalently coupled to the primary face of β-CD using 1,2,3-triazole moiety along with varying lengths of linker, were synthesized via copper-catalyzed azide-alkyl cycloaddition reaction. Benefited from the attached β-CD moiety, all these conjugates showed lower hydrophobicity (Alog P) compared with their parent compound GA. With the exception of per- O-methylated β-CD-GA conjugate ( 35), all other conjugates showed no significant cytotoxicity to MDCK cells, and these conjugates were then screened against A/WSN/33 (H1N1) virus using the cytopathic effect assay. The preliminary results indicated that six conjugates showed promising antiviral activity, and the C-3 and C-30 of GA could tolerate some modifications. Our findings suggested that GA could be used as a lead compound for the development of potential anti-influenza virus agents.
Graphical abstract
Highlights
-
•
Triterpene natural products are proposed as a new class of compounds for blocking influenza virus entry.
-
•
A total of 18 β-cyclodextrin-glycyrrhetinic acid conjugates were designed and synthesized via click chemistry.
-
•
The structure-activity relationship of their anti-influenza A/WSN/33 (H1N1) viruses was summarized.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus.
- Record: found
- Abstract: found
- Article: not found
The Pharmacological Activities of Licorice.
- Record: found
- Abstract: found
- Article: not found

