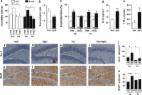
Depression is one of the most common psychiatric symptoms in Alzheimer's disease (AD), and considerable evidence indicates that major depressive disorder increases the risk of AD. 1, 2, 3 To date, however, the molecular mechanisms underlying the clinical association between depression and AD have remained elusive. Soluble oligomers of the amyloid-β peptide (AβOs) accumulate in the brains of AD patients and are increasingly recognized as the proximal neurotoxins responsible for synapse failure and memory deficits in AD. 4, 5 We have hypothesized that AβOs might be mechanistically linked to behavioral changes in AD. In order to test this hypothesis, mice were given a single intracerebroventricular (i.c.v.) injection of 10 pmol AβOs and were subsequently evaluated in the Porsolt forced swim test (FST) for assessment of depressive-like behavior. Compared with vehicle-injected control mice, AβO-injected mice exhibited a significant increase in immobility in the FST, both 24 h and 8 days after AβO injections (Figure 1a). Similar results were obtained when animals were assessed in the tail suspension test, another classical task to evaluate depressive-like behavior in rodents (Supplementary Figure S1a). AβO-induced immobility in the FST was blocked by anti-depressant (fluoxetine) treatment (Figure 1a). An important feature of depressive disorder is anhedonic behavior, including decreased interest for pleasurable sensorial experiences. Whereas vehicle-injected mice exhibited an expected preference for sucrose solution over plain water, AβO-injected mice did not exhibit such preference, indicating that AβOs instigate anhedonic behavior (Figure 1b). As memory deficit is the main clinical symptom of AD, we investigated the impact of AβOs on mice memory using the novel object recognition (OR) task. Results showed that 24 h after i.c.v. injection, AβO-treated mice spent equal amounts of time exploring both old (familiar) and new (novel) objects, indicating a deficit in declarative recognition memory, whereas vehicle-injected animals exhibited a significant preference for the novel object (Figure 1c). Treatment with fluoxetine prevented the memory deficit induced by AβOs (Figure 1c). Control measurements showed no changes in spontaneous exploratory or locomotor activities of fluoxetine-, saline-, vehicle- or AβO-injected animals during the training phase of the OR test (Supplementary Figure S1c–e). As the hippocampus is a key anatomical structure for OR memory, we sought to determine whether AβOs injected i.c.v. reached the hippocampus. Indeed, robust AβO immunoreactivity was verified using an anti-oligomer monoclonal antibody (NU4) 6 in hippocampi from AβO-injected mice, but not in hippocampi from control vehicle-injected animals (Supplementary Figure S1b). Together, these results indicate that AβOs have an acute impact on memory, learning and mood in mice, and that fluoxetine treatment prevented both cognitive impairment and depressive-like behavior induced by AβOs. The beneficial actions of fluoxetine have been partly ascribed to its anti-inflammatory effect. This led us to ask whether AβOs triggered an inflammatory response in the mouse brain. The brains of mice used in the tests described above were analyzed for levels of pro-inflammatory cytokines after i.c.v. injection of AβOs or vehicle. AβO-injected animals showed significantly elevated brain levels of interleukin-1β and tumor necrosis factor-α compared with vehicle-injected animals (Figure 1d, e). Sections from the hippocampus and cortex of AβO- or vehicle-injected mice were further immunostained for the presence of microglia (anti-Iba-1 antibody) and astrocytes (anti-GFAP antibody). Compared with vehicle-injected animals, AβO-injected mice showed markedly increased immunoreactivities for both Iba-1 and GFAP in the hippocampus and cortex 24 h after injection (Figure 1f–o, and Supplementary Figure 1f–o). The increase in glial cell numbers instigated by AβOs was blocked by fluoxetine treatment of the animals before AβO injection (Figure 1f–o, and Supplementary Figure 1f–o). The current findings establish that AβOs link memory impairment and depressive-like behavior in mice, providing molecular mechanistic support to clinical evidence connecting AD and depressive disorder. The impact of AβOs on mood, learning and memory, and its prevention by fluoxetine, can likely be attributed to the activation of inflammatory pathways (as shown here) and, possibly, to the deregulation of the serotonergic axis. The latter possibility is in line with the observation that pro-inflammatory cytokines impact serotonin metabolism 7, 8 and that increased serotonin levels are associated with lower brain Aβ levels in transgenic mouse models of AD and in humans. 9 Moreover, 5-HT1A and 5-HT2A receptors have been reported to be reduced in post-mortem AD brain, 10 and 5-HT1A receptors have been found to be reduced in vivo in AD. 11 Brain disturbances that place a person at risk for developing depression and AD are still largely unknown. By revealing that AβOs underlie both cognitive and depressive-like symptoms in mice, our results suggest a mechanism by which elevated brain levels of AβOs may be linked to changes in cognition and mood in AD.