- Record: found
- Abstract: found
- Article: found
Arrangement and symmetry of the fungal E3BP-containing core of the pyruvate dehydrogenase complex
Read this article at
Abstract
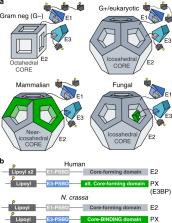
The pyruvate dehydrogenase complex (PDC) is a multienzyme complex central to aerobic respiration, connecting glycolysis to mitochondrial oxidation of pyruvate. Similar to the E3-binding protein (E3BP) of mammalian PDC, PX selectively recruits E3 to the fungal PDC, but its divergent sequence suggests a distinct structural mechanism. Here, we report reconstructions of PDC from the filamentous fungus Neurospora crassa by cryo-electron microscopy, where we find protein X (PX) interior to the PDC core as opposed to substituting E2 core subunits as in mammals. Steric occlusion limits PX binding, resulting in predominantly tetrahedral symmetry, explaining previous observations in Saccharomyces cerevisiae. The PX-binding site is conserved in (and specific to) fungi, and complements possible C-terminal binding motifs in PX that are absent in mammalian E3BP. Consideration of multiple symmetries thus reveals a differential structural basis for E3BP-like function in fungal PDC.
Abstract
The pyruvate dehydrogenase complex (PDC) is a multienzyme complex connecting glycolysis to mitochondrial oxidation of pyruvate. Cryo-EM analysis of PDC from Neurospora crassa reveals localization of fungi-specific protein X (PX) and confirms that it functions like the mammalian E3BP, recruiting the E3 component of PDC.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Regulation of pyruvate metabolism and human disease
- Record: found
- Abstract: found
- Article: not found
A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence.
- Record: found
- Abstract: found
- Article: not found
The pyruvate dehydrogenase complexes: structure-based function and regulation.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
