- Record: found
- Abstract: found
- Article: found
Alp7/TACC recruits kinesin-8–PP1 to the Ndc80 kinetochore protein for timely mitotic progression and chromosome movement
Read this article at
ABSTRACT
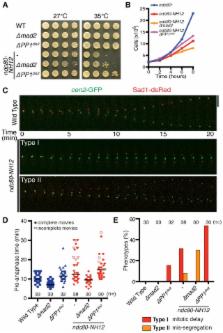
Upon establishment of proper kinetochore–microtubule attachment, the spindle assembly checkpoint (SAC) must be silenced to allow onset of anaphase, which is when sister chromatids segregate equally to two daughter cells. However, how proper kinetochore–microtubule attachment leads to timely anaphase onset remains elusive. Furthermore, the molecular mechanisms of chromosome movement during anaphase A remain unclear. In this study, we show that the fission yeast Alp7/TACC protein recruits a protein complex consisting of the kinesin-8 (Klp5–Klp6) and protein phosphatase 1 (PP1) to the kinetochore upon kinetochore–microtubule attachment. Accumulation of this complex at the kinetochore, on the one hand, facilitates SAC inactivation through PP1, and, on the other hand, accelerates polewards chromosome movement driven by the Klp5–Klp6 motor. We identified an alp7 mutant that had specific defects in binding to the Klp5–Klp6–PP1 complex but with normal localisation to the microtubule and kinetochore. Consistent with our proposition, this mutant shows delayed anaphase onset and decelerated chromosome movement during anaphase A. We propose that the recruitment of kinesin-8–PP1 to the kinetochore through Alp7/TACC interaction plays a crucial role in regulation of timely mitotic progression and chromosome movement during anaphase A.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
The spindle-assembly checkpoint in space and time.
- Record: found
- Abstract: found
- Article: not found
The conserved KMN network constitutes the core microtubule-binding site of the kinetochore.
- Record: found
- Abstract: found
- Article: not found