- Record: found
- Abstract: found
- Article: not found
PGC-1α Deficiency Causes Multi-System Energy Metabolic Derangements: Muscle Dysfunction, Abnormal Weight Control and Hepatic Steatosis
Read this article at
Abstract
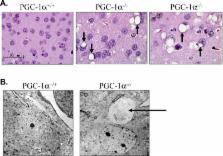
The gene encoding the transcriptional coactivator peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) was targeted in mice. PGC-1α null (PGC-1α −/−) mice were viable. However, extensive phenotyping revealed multi-system abnormalities indicative of an abnormal energy metabolic phenotype. The postnatal growth of heart and slow-twitch skeletal muscle, organs with high mitochondrial energy demands, is blunted in PGC-1α −/− mice. With age, the PGC-1α −/− mice develop abnormally increased body fat, a phenotype that is more severe in females. Mitochondrial number and respiratory capacity is diminished in slow-twitch skeletal muscle of PGC-1α −/− mice, leading to reduced muscle performance and exercise capacity. PGC-1α −/− mice exhibit a modest diminution in cardiac function related largely to abnormal control of heart rate. The PGC-1α −/− mice were unable to maintain core body temperature following exposure to cold, consistent with an altered thermogenic response. Following short-term starvation, PGC-1α −/− mice develop hepatic steatosis due to a combination of reduced mitochondrial respiratory capacity and an increased expression of lipogenic genes. Surprisingly, PGC-1α −/− mice were less susceptible to diet-induced insulin resistance than wild-type controls. Lastly, vacuolar lesions were detected in the central nervous system of PGC-1α −/− mice. These results demonstrate that PGC-1α is necessary for appropriate adaptation to the metabolic and physiologic stressors of postnatal life.
Abstract
Eliminating the activity of the gene PGC-1 α in mice reveals its role in post-natal metabolism and provides a link to obesity and some intriguing differences with another report of this knockout
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis.
- Record: found
- Abstract: not found
- Article: not found
Transcriptional regulatory circuits controlling mitochondrial biogenesis and function.
- Record: found
- Abstract: found
- Article: not found
