- Record: found
- Abstract: found
- Article: not found
Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease
Read this article at
Abstract
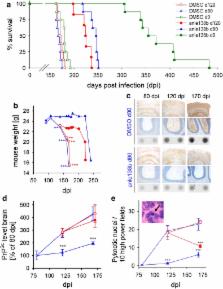
In neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and prion diseases, deposits of aggregated disease-specific proteins are found. Oligomeric aggregates are presumed to be the key neurotoxic agent. Here we describe the novel oligomer modulator anle138b [3-(1,3-benzodioxol-5-yl)-5-(3-bromophenyl)-1 H-pyrazole], an aggregation inhibitor we developed based on a systematic high-throughput screening campaign combined with medicinal chemistry optimization. In vitro, anle138b blocked the formation of pathological aggregates of prion protein (PrP Sc) and of α-synuclein (α-syn), which is deposited in PD and other synucleinopathies such as dementia with Lewy bodies (DLB) and multiple system atrophy (MSA). Notably, anle138b strongly inhibited all prion strains tested including BSE-derived and human prions. Anle138b showed structure-dependent binding to pathological aggregates and strongly inhibited formation of pathological oligomers in vitro and in vivo both for prion protein and α-synuclein. Both in mouse models of prion disease and in three different PD mouse models, anle138b strongly inhibited oligomer accumulation, neuronal degeneration, and disease progression in vivo. Anle138b had no detectable toxicity at therapeutic doses and an excellent oral bioavailability and blood–brain-barrier penetration. Our findings indicate that oligomer modulators provide a new approach for disease-modifying therapy in these diseases, for which only symptomatic treatment is available so far. Moreover, our findings suggest that pathological oligomers in neurodegenerative diseases share structural features, although the main protein component is disease-specific, indicating that compounds such as anle138b that modulate oligomer formation by targeting structure-dependent epitopes can have a broad spectrum of activity in the treatment of different protein aggregation diseases.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders.
- Record: found
- Abstract: found
- Article: not found
A highly reproducible rotenone model of Parkinson's disease.
- Record: found
- Abstract: not found
- Article: not found
