- Record: found
- Abstract: found
- Article: found
The prognostic impact of programmed cell death ligand 1 and human leukocyte antigen class I in pancreatic cancer
Read this article at
Abstract
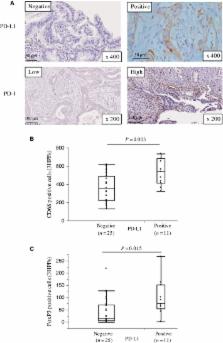
Pancreatic ductal adenocarcinoma ( PDA) is associated with an immunosuppressive tumor‐microenvironment ( TME) that supports the growth of tumors and mediates tumors enabling evasion of the immune system. Expression of programmed cell death ligand 1 ( PD‐L1) and loss of human leukocyte antigen ( HLA) class I on tumor cells are methods by which tumors escape immunosurveillance. We examined immune cell infiltration, the expression of PD‐L1 and HLA class I by PDA cells, and the correlation between these immunological factors and clinical prognosis. PDA samples from 36 patients were analyzed for HLA class I, HLA‐ DR, PD‐L1, PD‐1, CD4, CD8, CD56, CD68, and FoxP3 expression by immunohistochemistry. The correlations between the expression of HLA class I, HLA‐ DR, PD‐L1 or PD‐1 and the pattern of tumor infiltrating immune cells or the patients’ prognosis were assessed. PD‐L1 expression correlated with tumor infiltration by CD68 + and FoxP3 + cells. Low HLA class I expression was an only risk factor for poor survival. PD‐L1 negative and HLA class I high‐expressing PDA was significantly associated with higher numbers of infiltrating CD8 + T cells in the TME, and a better prognosis. Evaluation of both PD‐L1 and HLA class I expression by PDA may be a good predictor of prognosis for patients. HLA class I expression by tumor cells should be evaluated when selecting PDA patients who may be eligible for treatment with PD‐1/ PD‐L1 immune checkpoint blockade therapies.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
The blockade of immune checkpoints in cancer immunotherapy.
- Record: found
- Abstract: found
- Article: not found
In search of the ‘missing self’: MHC molecules and NK cell recognition
- Record: found
- Abstract: found
- Article: not found