- Record: found
- Abstract: found
- Article: found
Investigation of market herbal products regulated under different categories: How can HPTLC help to detect quality problems?
Read this article at
Abstract
Background: Herbal products regulated under different categories were found to be of different quality. This has been demonstrated by the increasing number of reports on the quality of herbal products in the scientific literature. Proper identification is an effective way to address this concerning issue early on in a products’ manufacturing process.
Objectives: To assess the quality of milk thistle, coneflower and black cohosh herbal drugs, preparations and products commercialized under different regulatory categories, and to illustrate the usefulness of HPTLC as a tool for evaluating quality.
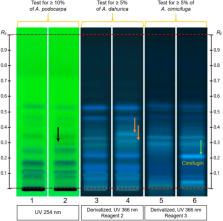
Methods: HPTLC methods were adapted from the European Pharmacopeia’s monographs for milk thistle fruits, black cohosh and purple coneflower. Additional detection modes beyond those described in the monographs were employed, and the entire HPTLC fingerprints were used for examination of identity and purity of the investigated samples.
Results: All products regulated as Traditional Herbal Medicinal Products were shown to be of high quality: their fingerprints were consistent and without unexpected zones. A significant number of food supplements show quality issues (mainly adulterations): 52.4% of milk thistle, 33.3% of coneflower, and 45.5% of black cohosh products. The same was observed in 66.6% of black cohosh herbal drugs and preparations.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Quality of herbal medicines: challenges and solutions.
- Record: found
- Abstract: found
- Article: not found
DNA Barcoding for the Identification of Botanicals in Herbal Medicine and Dietary Supplements: Strengths and Limitations.
- Record: found
- Abstract: found
- Article: not found