- Record: found
- Abstract: found
- Article: not found
Increase of circulating endocan over sepsis follow-up is associated with progression into organ dysfunction
Read this article at
Abstract
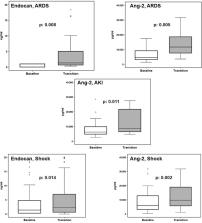
How circulating inflammatory mediators change upon sepsis progression has not been studied. We studied the follow-up changes of circulating vasoactive peptides and cytokines until the improvement or the worsening of a patient and progression into specific organ dysfunctions. In a prospective study, concentrations of tumor necrosis factor-alpha (TNFα), interleukin (IL)-6, IL-8, IL-10, interferon-gamma (IFNγ), endocan and angiopoietin-2 (Ang-2) were measured in serum by an enzyme immunoassay in 175 patients at baseline; this was repeated within 24 h upon progression into new organ dysfunction ( n = 141) or improvement ( n = 34). Endocan and Ang-2 were the only parameters that were significantly increased among patients who worsened. Any increase of endocan was associated with worsening with odds ratio 16.65 ( p < 0.0001). This increase was independently associated with progression into acute respiratory distress syndrome (ARDS) as shown after logistic regression analysis (odds ratio 2.91, p: 0.002). Changes of circulating cytokines do not mediate worsening of the critically ill patients. Instead endocan and Ang2 are increased and this may be interpreted as a key-playing role in the pathogenesis of ARDS and septic shock. Any increase of endocan is a surrogate of worsening of the clinical course.
Related collections
Most cited references12
- Record: found
- Abstract: found
- Article: not found
The international sepsis forum consensus conference on definitions of infection in the intensive care unit.
- Record: found
- Abstract: found
- Article: not found
Reversal of immunoparalysis in humans in vivo: a double-blind, placebo-controlled, randomized pilot study.
- Record: found
- Abstract: found
- Article: not found
