- Record: found
- Abstract: found
- Article: found
A heat-shocked melanoma cell lysate vaccine enhances tumor infiltration by prototypic effector T cells inhibiting tumor growth
Read this article at
Abstract
Background
Immune checkpoint blocker (ICB) therapy has shown survival benefits for some patients with cancer. Nevertheless, many individuals remain refractory or acquire resistance to treatment, motivating the exploration of complementary immunotherapies. Accordingly, cancer vaccines offer an attractive alternative. Optimal delivery of multiple tumor-associated antigens combined with potent adjuvants seems to be crucial for vaccine effectiveness.
Methods
Here, a prototype for a generic melanoma vaccine, named TRIMELVax, was tested using B16F10 mouse melanoma model. This vaccine is made of heat shock-treated tumor cell lysates combined with the Concholepas concholepas hemocyanin as adjuvant.
Results
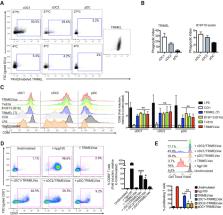
While B16F10 lysate provides appropriate melanoma-associated antigens, both a generic human melanoma cell lysate and hemocyanin adjuvant contributes with danger signals promoting conventional dendritic type 1 cells (cDC1), activation, phagocytosis and effective antigen cross-presentation. TRIMELVax inhibited tumor growth and increased mice survival, inducing cellular and humoral immune responses. Furthermore, this vaccine generated an increased frequency of intratumor cDC1s but not conventional type 2 dendritic cells (cDC2s). Augmented infiltration of CD3 +, CD4 + and CD8 + T cells was also observed, compared with anti-programmed cell death protein 1 (PD-1) monotherapy, while TRIMELVax/anti-PD-1 combination generated higher tumor infiltration of CD4 + T cells. Moreover, TRIMELVax promoted an augmented proportion of PD-1 lo CD8 + T cells in tumors, a phenotype associated with prototypic effector cells required for tumor growth control, preventing dysfunctional T-cell accumulation.
Conclusions
The therapeutic vaccine TRIMELVax efficiently controls the weakly immunogenic and aggressive B16F10 melanoma tumor growth, prolonging tumor-bearing mice survival even in the absence of ICB. The strong immunogenicity shown by TRIMELVax encourages clinical studies in patients with melanoma.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors.

- Record: found
- Abstract: found
- Article: found
Cold Tumors: A Therapeutic Challenge for Immunotherapy
- Record: found
- Abstract: found
- Article: not found
Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.