- Record: found
- Abstract: found
- Article: found
Isolation and characterization of equine endometrial mesenchymal stromal cells
Read this article at
Abstract
Background
Equine mesenchymal stromal/stem cells (MSCs) are most commonly harvested from bone marrow (BM) or adipose tissue, requiring the use of surgical procedures. By contrast, the uterus can be accessed nonsurgically, and may provide a more readily available cell source. While human endometrium is known to harbor mesenchymal precursor cells, MSCs have not been identified in equine endometrium. This study reports the isolation, culture, and characterization of MSCs from equine endometrium.
Methods
The presence of MSC and pericyte markers in endometrial sections was determined using immunohistochemistry. Stromal cells were harvested and cultured after separation of epithelial cells from endometrial fragments using Mucin-1-bound beads. For comparison, MSCs were also harvested from BM. The expression of surface markers in endometrial and BM-derived MSCs was characterized using flow cytometry and quantitative polymerase chain reaction. MSCs were differentiated in vitro into adipogenic, chondrogenic, osteogenic, and smooth muscle lineages.
Results
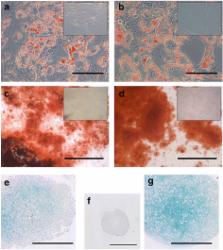
Typical markers of MSCs (CD29, CD44, CD90, and CD105) and pericytes (NG2 and CD146) were localized in the equine endometrium. Both endometrial and BM MSCs grew clonally and robustly expressed MSC and pericyte markers in culture while showing greatly reduced or negligible expression of hematopoietic markers (CD45, CD34) and MHC-II. Additionally, both endometrial and BM MSCs differentiated into adipogenic, osteogenic, and chondrogenic lineages in vitro, and endometrial MSCs had a distinct ability to undergo smooth muscle differentiation.
Conclusions
We have demonstrated for the first time the presence of cells in equine endometrium that fulfill the definition of MSCs. The equine endometrium may provide an alternative, easily accessible source of MSCs, not only for therapeutic regeneration of the uterus, but also for other tissues where MSCs from other sources are currently being used therapeutically.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Mesenchymal progenitor cells in human umbilical cord blood.

- Record: found
- Abstract: found
- Article: not found