- Record: found
- Abstract: found
- Article: found
Polysulfide-1-oxides react with peroxyl radicals as quickly as hindered phenolic antioxidants and do so by a surprising concerted homolytic substitution†

Read this article at
Abstract
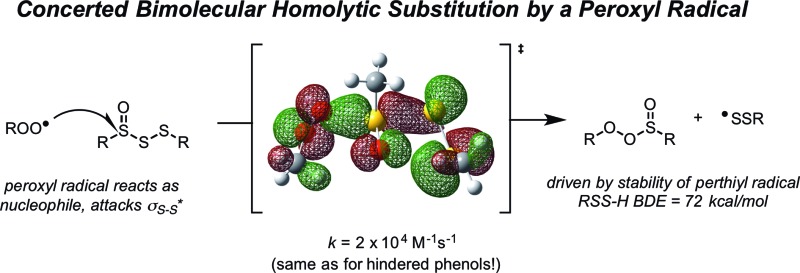
Abstract
Polysulfides are important additives to a wide variety of industrial and consumer
products and figure prominently in the chemistry and biology of garlic and related
medicinal plants. Although their antioxidant activity in biological contexts has received
only recent attention, they have long been ascribed ‘secondary antioxidant’ activity
in the chemical industry, where they are believed to react with the hydroperoxide
products of autoxidation to slow the auto-initiation of new autoxidative chain reactions.
Herein we demonstrate that the initial products of trisulfide oxidation, trisulfide-1-oxides,
are surprisingly reactive ‘primary antioxidants’, which slow autoxidation by trapping
chain-carrying peroxyl radicals. In fact, they do so with rate constants (
k = 1–2 × 10
4 M
–1 s
–1 at 37 °C) that are indistinguishable from those of the most common primary antioxidants,
i.e. hindered phenols, such as BHT. Experimental and computational studies demonstrate
that the reaction occurs by a concerted bimolecular homolytic substitution (S
H
2), liberating a perthiyl radical – which is
ca. 16 kcal mol
–1 more stable than a peroxyl radical. Interestingly, the (electrophilic) peroxyl radical
nominally reacts as a nucleophile – attacking the
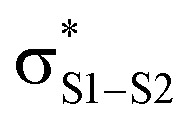 of the trisulfide-1-oxide – a role hitherto suspected only for its reactions at metal
atoms. The analogous reactions of trisulfides are readily reversible and not kinetically
competent to inhibit hydrocarbon autoxidation, consistent with the longstanding view
that organosulfur compounds must be oxidized to afford significant antioxidant activity.
The reactivity of trisulfides and their oxides are contrasted with what is known of
their shorter cousins and predictions are made and tested with regards to the reactivity
of higher polysulfides and their 1-oxides – the insights from which may be exploited
in the design of future antioxidants.
of the trisulfide-1-oxide – a role hitherto suspected only for its reactions at metal
atoms. The analogous reactions of trisulfides are readily reversible and not kinetically
competent to inhibit hydrocarbon autoxidation, consistent with the longstanding view
that organosulfur compounds must be oxidized to afford significant antioxidant activity.
The reactivity of trisulfides and their oxides are contrasted with what is known of
their shorter cousins and predictions are made and tested with regards to the reactivity
of higher polysulfides and their 1-oxides – the insights from which may be exploited
in the design of future antioxidants.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Persulfide reactivity in the detection of protein s-sulfhydration.
- Record: found
- Abstract: found
- Article: not found
Free-radical repair by a novel perthiol: reversible hydrogen transfer and perthiyl radical formation.
- Record: found
- Abstract: found
- Article: not found
The mechanism of radical-trapping antioxidant activity of plant-derived thiosulfinates.
Author and article information
Notes
†Electronic supplementary information (ESI) available: NMR spectra of new compounds, KIE experiments, details of LFP experiments and optimized geometries and energies for computational results. See DOI: 10.1039/c6sc01434h
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.