- Record: found
- Abstract: found
- Article: found
Nematode infection in liver of the fish Gymnotus inaequilabiatus (Gymnotiformes: Gymnotidae) from the Pantanal Region in Brazil: pathobiology and inflammatory response
Read this article at
Abstract
Background
A survey on endoparasitic helminths from freshwater fishes in the Pantanal Region (Mato Grosso do Sul, Brazil) revealed the occurrence of third-larval stage of the nematode Brevimulticaecum sp. (Heterocheilidae) in most organs of Gymnotus inaequilabiatus (Gymnotidae) also known by the local name tuvira. The aim of the present study was to examine Brevimulticaecum sp.-infected tuvira liver at the ultrastructural level and clarify the nature of granulomas and the cellular elements involved in the immune response to nematode larvae.
Methods
Thirty-eight adult specimens of tuvira from Porto Morrinho, were acquired in January and March 2016. Infected and uninfected liver tissues were fixed and prepared for histological and ultrastructure investigations.
Results
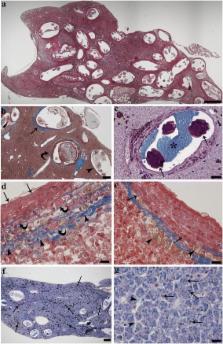
The prevalence of infection of tuvira liver by the nematode larvae was 95 %, with an intensity of infection ranging from 4 to 343 larvae (mean ± SD: 55.31 ± 73.94 larvae per liver). In livers with high numbers of nematode larvae, almost entire hepatic tissue was occupied by the parasites. Hepatocytes showed slight to mild degenerative changes and accumulation of pigments. Parasite larvae were surrounded by round to oval granulomas, the result of focal host tissue response to the infection. Each granuloma was typically formed by three concentric layers: an outer layer of fibrous connective tissue with thin elongated fibroblasts; a middle layer of mast cells entrapped in a thin fibroblast-connective mesh; and an inner layer of densely packed epithelioid cells, displaying numerous desmosomes between each other. Numerous macrophage aggregates occurred in the granulomas and in the parenchyma.
Conclusions
Our results in tuvira showed that the larvae were efficiently sequestered within the granulomas, most of the inflammatory components were confined within the thickness of the granuloma, and the parenchyma was relatively free of immune cells and without fibrosis. Presumably this focal encapsulation of the parasites permits uninfected portions of liver to maintain its functions and allows the survival of the host.
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
The zebrafish as a model to study intestinal inflammation.
- Record: found
- Abstract: found
- Article: not found
Immune system and immune responses in fish and their role in comparative immunity study: a model for higher organisms.
- Record: found
- Abstract: found
- Article: not found