- Record: found
- Abstract: found
- Article: found
Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts
Read this article at
Abstract
Background
Hepatocellular carcinoma (HCC) remains a global challenge due to its high morbidity and mortality rates as well as poor response to treatment. The communication between tumor-derived elements and stroma plays a critical role in facilitating cancer progression of HCC. Exosomes are small extracellular vesicles (EVs) that are released from the cells upon fusion of multivesicular bodies with the plasma membrane. There is emerging evidence indicating that exosomes play a central role in cell-to-cell communication. Much attention has been paid to exosomes since they are found to transport bioactive proteins, messenger RNA (mRNAs) and microRNA (miRNAs) that can be transferred in active form to adjacent cells or to distant organs. However, the mechanisms underlying such cancer progression remain largely unexplored.
Methods
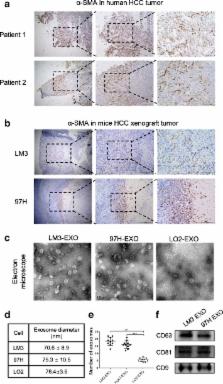
Exosomes were isolated by differential ultracentrifugation from conditioned medium of HCC cells and identified by electron microscopy and Western blotting analysis. Hepatic stellate cells (HSCs) were treated with different concentrations of exosomes, and the activation of HSCs was analyzed by Western blotting analysis, wound healing, migration assay, Edu assay, CCK-8 assay and flow cytometry. Moreover, the different miRNA levels of exosomes were tested by real-time quantitative PCR (RT-PCR). The angiogenic ability of activated HSCs was analyzed by qRT-PCR, CCK-8 assay and tube formation assay. In addition, the abnormal lipid metabolism of activated HSCs was analyzed by Western blotting analysis and Oil Red staining. Finally, the relationship between serum exosomal miRNA-21 and prognosis of HCC patients was evaluated.
Results
We showed that HCC cells exhibited a great capacity to convert normal HSCs to cancer-associated fibroblasts (CAFs). Moreover, our data revealed that HCC cells secreted exosomal miRNA-21 that directly targeted PTEN, leading to activation of PDK1/AKT signaling in HSCs. Activated CAFs further promoted cancer progression by secreting angiogenic cytokines, including VEGF, MMP2, MMP9, bFGF and TGF-β. Clinical data indicated that high level of serum exosomal miRNA-21 was correlated with greater activation of CAFs and higher vessel density in HCC patients.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Modulation of K-Ras-dependent lung tumorigenesis by MicroRNA-21.
- Record: found
- Abstract: found
- Article: not found
The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence.
- Record: found
- Abstract: found
- Article: found