- Record: found
- Abstract: found
- Article: found
Effect of high-dose simvastatin on cognitive, neuropsychiatric, and health-related quality-of-life measures in secondary progressive multiple sclerosis: secondary analyses from the MS-STAT randomised, placebo-controlled trial
Summary
Background
In the 24-month MS-STAT phase 2 trial, we showed that high-dose simvastatin significantly reduced the annualised rate of whole brain atrophy in patients with secondary progressive multiple sclerosis (SPMS). We now describe the results of the MS-STAT cognitive substudy, in which we investigated the treatment effect on cognitive, neuropsychiatric, and health-related quality-of-life (HRQoL) outcome measures.
Methods
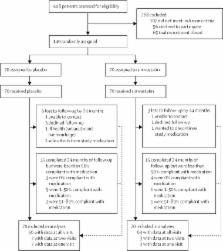
We did a secondary analysis of MS-STAT, a 24-month, double-blind, controlled trial of patients with SPMS done at three neuroscience centres in the UK between Jan 28, 2008, and Nov 4, 2011. Patients were randomly assigned (1:1) to either 80 mg simvastatin (n=70) or placebo (n=70). The cognitive assessments done were the National Adult Reading Test, Wechsler Abbreviated Scale of Intelligence, Graded Naming Test, Birt Memory and Information Processing Battery (BMIPB), Visual Object and Space Perception battery (cube analysis), Frontal Assessment Battery (FAB), and Paced Auditory Serial Addition Test. Neuropsychiatric status was assessed using the Hamilton Depression Rating Scale and the Neuropsychiatric Inventory Questionnaire. HRQoL was assessed using the self-reported 36-Item Short Form Survey (SF-36) version 2. Assessments were done at study entry, 12 months, and 24 months. Patients, treating physicians, and outcome assessors were masked to treatment allocation. Analyses were by intention to treat. MS-STAT is registered with ClinicalTrials.gov, number NCT00647348.
Findings
Baseline assessment revealed impairments in 60 (45%) of 133 patients on the test of frontal lobe function (FAB), and in between 13 (10%) and 43 (33%) of 130 patients in tests of non-verbal and verbal memory (BMIPB). Over the entire trial, we noted significant worsening on tests of verbal memory (T score decline of 5·7 points, 95% CI 3·6–7·8; p<0·0001) and non-verbal memory (decline of 6·8 points, 4·8–8·7; p<0·0001). At 24 months, the FAB score was 1·2 points higher in the simvastatin-treated group than in the placebo group (95% CI 0·2–2·3). The simvastatin group also had a 2·5 points better mean physical component score of the SF-36 (95% CI 0·3–4·8; p=0·028). A treatment effect was not noted for any other outcomes.
Interpretation
To our knowledge, this SPMS cohort is the largest studied to date with comprehensive longitudinal cognitive, neuropsychiatric, and HRQoL assessments. We found evidence of a positive effect of simvastatin on frontal lobe function and a physical quality-of-life measure. Although we found no effect of simvastatin on the other outcome measures, these potential effects warrant confirmation and underline the importance of fully assessing cognition and quality of life in progressive multiple sclerosis treatment trials.
Funding
The Moulton Foundation, the Berkeley Foundation, the Multiple Sclerosis Trials Collaboration, the Rosetrees Trust, a personal contribution from A W Pidgley CBE, and the National Institute for Health Research University College London Hospitals Biomedical Research Centre and University College London.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning.
- Record: found
- Abstract: found
- Article: not found
Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes
- Record: found
- Abstract: found
- Article: not found