- Record: found
- Abstract: found
- Article: found
3D bioprinting using a new photo-crosslinking method for muscle tissue restoration
Read this article at
Abstract
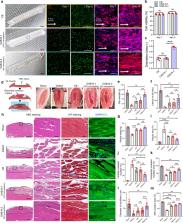
Three-dimensional (3D) bioprinting is a highly effective technique for fabricating cell-loaded constructs in tissue engineering. However, the versatility of fabricating precise and complex cell-loaded hydrogels is limited owing to the poor crosslinking ability of cell-containing hydrogels. Herein, we propose an optic-fiber-assisted bioprinting (OAB) process to efficiently crosslink methacrylated hydrogels. By selecting appropriate processing conditions for the photo-crosslinking technique, we fabricated biofunctional cell-laden structures including methacrylated gelatin (Gelma), collagen, and decellularized extracellular matrix. To apply the method to skeletal muscle regeneration, cell-laden Gelma constructs were processed with a functional nozzle having a topographical cue and an OAB process that could induce a uniaxial alignment of C2C12 and human adipose stem cells (hASCs). Significantly higher degrees of cell alignment and myogenic activities in the cell-laden Gelma structure were observed compared with those in the cell construct that was printed using a conventional crosslinking method. Moreover, an in vivo regenerative potential was observed in volumetric muscle defects in a mouse model. The hASC-laden construct significantly induced greater muscle regeneration than the cell construct without topographical cues. Based on the results, the newly designed bioprinting process can prove to be highly effective in fabricating biofunctional cell-laden constructs for various tissue engineering applications.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels.
- Record: found
- Abstract: found
- Article: not found