- Record: found
- Abstract: found
- Article: found
Caecal infusion of the short‐chain fatty acid propionate affects the microbiota and expression of inflammatory cytokines in the colon in a fistula pig model
Read this article at
Summary
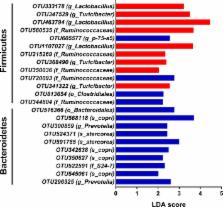
Short‐chain fatty acids ( SCFAs), particularly butyrate, are known to suppress inflammation, and regulate the gut bacterial ecology. However, little is known about propionate. We report here that propionate infusion in the caecum dramatically affected the structure of colonic microbiota of pigs based on 16s rRNA high‐throughput sequencing. Sixteen pig models were perfused with saline or sodium propionate by a fistula in the caecum. At d 28, all pigs were slaughtered for analysing bacterial metabolites, colonic microbiota and the expression of genes related to inflammation. The results showed that caecal infusion of sodium propionate increased the concentration of propionate and decreased the butyrate concentration in colonic content. For biogenic amines, the tyramine concentration was increased, while the concentration of cadaverine was decreased by infusion of sodium propionate. Furthermore, at the level of phylum, propionate increased the abundance of Bacteroidetes and reduced the abundance of Firmicutes. Prevotella and Bacteroides counts were increased, while Turicibacter abundance was decreased at the level of genus. Real‐time qPCR showed that the expression of NF‐κB and IL‐18 was upregulated by propionate infusion, whereas no significant differences were observed for the expression of other genes related to inflammatory processes. Taken together, these results provide a new evidence for the role of short‐chain fatty acid propionate on the composition of microbial community and inflammatory cytokines.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Has the microbiota played a critical role in the evolution of the adaptive immune system?
- Record: found
- Abstract: found
- Article: not found
Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease.
- Record: found
- Abstract: found
- Article: not found