- Record: found
- Abstract: found
- Article: found
Nano-selenium and its nanomedicine applications: a critical review
Abstract
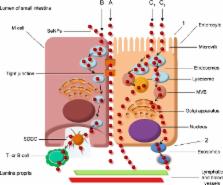
Traditional supplements of selenium generally have a low degree of absorption and increased toxicity. Therefore, it is imperative to develop innovative systems as transporters of selenium compounds, which would raise the bioavailability of this element and allow its controlled release in the organism. Nanoscale selenium has attracted a great interest as a food additive especially in individuals with selenium deficiency, but also as a therapeutic agent without significant side effects in medicine. This review is focused on the incorporation of nanotechnological applications, in particular exploring the possibilities of a more effective way of administration, especially in selenium-deficient organisms. In addition, this review summarizes the survey of knowledge on selenium nanoparticles, their biological effects in the organism, advantages, absorption mechanisms, and nanotechnological applications for peroral administration.
Most cited references185
- Record: found
- Abstract: found
- Article: not found
Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma.
- Record: found
- Abstract: found
- Article: not found
Nanoparticle-based targeted drug delivery.
- Record: found
- Abstract: found
- Article: not found