- Record: found
- Abstract: found
- Article: not found
Immunohistochemical Analysis of Histone H3 Modifications in Germ Cells during Mouse Spermatogenesis
Read this article at
Abstract
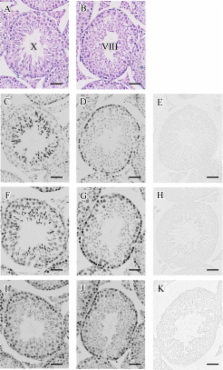
Histone modification has been implicated in the regulation of mammalian spermatogenesis. However, the association of differently modified histone H3 with a specific stage of germ cells during spermatogenesis is not fully understood. In this study, we examined the localization of variously modified histone H3 in paraffin-embedded sections of adult mouse testis immunohistochemically, focusing on acetylation at lysine 9 (H3K9ac), lysine 18 (H3K18ac), and lysine 23 (H3K23ac); tri-methylation at lysine 4 (H3K4me3) and lysine 27 (H3K27me3); and phosphorylation at serine 10 (H3S10phos). As a result, we found that there was a significant fluctuation in the modifications; in spermatogonia, the stainings for H3K9ac, H3K18ac, and H3K23ac were strong while that for H3K4me3 was weak. In spermatocytes, the stainings for H3K9ac, H3K18ac, H3K23ac, and H3K4me3 were reduced in the preleptotene to pachytene stage, but in diplotene stage the stainings for H3K18ac, H3K23ac, and H3K4me3 seemed to become intense again. The staining for H3K27me3 was nearly constant throughout these stages. In the ensuing spermiogenesis, a dramatic acetylation and methylation of histone H3 was found in the early elongated spermatids and then almost all signals disappeared in the late elongated spermatids, in parallel with the replacement from histones to protamines. In addition, we confirmed that the staining of histone H3S10phos was exclusively associated with mitotic and meiotic cell division. Based upon the above results, we indicated that the modification pattern of histone H3 is subject to dynamic change and specific to a certain stage of germ cell differentiation during mouse spermatogenesis.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Lineage-specific polycomb targets and de novo DNA methylation define restriction and potential of neuronal progenitors.
- Record: found
- Abstract: found
- Article: not found
Histone acetylation and an epigenetic code.
- Record: found
- Abstract: found
- Article: not found
