- Record: found
- Abstract: found
- Article: found
Tandem Isotope Therapy with 225Ac- and 177Lu-PSMA-617 in a Murine Model of Prostate Cancer
Read this article at
Visual Abstract
Abstract
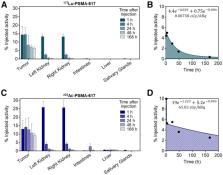
Radionuclide therapy targeting prostate-specific membrane antigen (PSMA) is a promising option for metastatic castration-resistant prostate cancer. Clinical experience using 177Lu or 225Ac has demonstrated encouraging treatment responses; however, responses are not durable. Dual-isotope combinations, or “tandem” approaches, may improve tolerability while retaining a high tumor dose. In this study, we directly compared α- versus β-particle treatment, as well as a combination thereof, at different stages of disease in a murine model of disseminated prostate cancer. Methods: First, to determine comparable injected activities from 177Lu- and 225Ac-PSMA-617, ex vivo biodistribution studies were performed at 5 time points after treatment of C4-2 subcutaneous tumor–bearing NSG mice. To establish a more representative model of metastatic prostate cancer, NSG mice were inoculated with luciferase-expressing C4-2 cells in the left ventricle, leading to disseminated visceral and bone lesions. At either 3 or 5 wk after inoculation, the mice were treated with equivalent tumor dose–depositing activities of 177Lu- or 225Ac-PSMA-617 alone or in combination (35 MBq of 177Lu, 40 kBq of 225Ac, or 17 MBq of 177Lu + 20 kBq 225Ac; 10/group). Disease burden was assessed by weekly bioluminescence imaging. Treatment efficacy was evaluated using whole-body tumor burden and overall survival. Results: The ex vivo biodistribution studies revealed that 35 MBq of 177Lu and 40 kBq of 225Ac yield equivalent absorbed tumor doses in a subcutaneous C4-2 model. The disease burden of mice treated at 3 wk after inoculation (microscopic disease) with 177Lu was not significantly different from that of untreated mice. However, 225Ac-PSMA-617 both as a single agent and in combination with 177Lu (17 MBq of 177Lu + 20 kBq of 225Ac) were associated with significant whole-body tumor growth retardation and survival benefit (overall survival, 8.3 wk for nontreatment, 9.4 wk for 177Lu, 15.3 wk for 225Ac alone, and 14.1 wk for tandem therapy). When treated at 5 wk after inoculation (macroscopic disease), all treatment groups showed retarded tumor growth and improved survival, with no significant differences between 225Ac alone and administration of half the 225Ac activity in tandem with 177Lu (overall survival, 7.9 wk for nontreatment, 10.3 wk for 177Lu, 14.6 wk for 225Ac alone, and 13.2 wk for tandem therapy). Conclusion: Treatment of a disseminated model of prostate cancer with simultaneous 225Ac- and 177Lu-PSMA-617 results in significantly decreased tumor growth compared with 177Lu, which was ineffective as a single agent against microscopic lesions. Mice treated later in the disease progression and bearing macroscopic, millimeter-sized lesions experienced significant tumor growth retardation and survival benefit in both monoisotopic and tandem regimens of 177Lu and 225Ac. Although the greatest benefits were observed with the single agent 225Ac, the tandem arm experienced no significant difference in disease burden or survival benefit, suggesting that the reduced activity of 225Ac was adequately compensated in the tandem arm. The superior therapeutic efficacy of 225Ac in this model suggests a preference for α-emitters alone, or possibly in combination, in the microscopic disease setting.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
[ 177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study
- Record: found
- Abstract: found
- Article: not found
German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients.
- Record: found
- Abstract: found
- Article: not found
