- Record: found
- Abstract: found
- Article: found
Kinetics and thermodynamics of enzymatic decarboxylation of α,β-unsaturated acid: a theoretical study†
Read this article at
Abstract
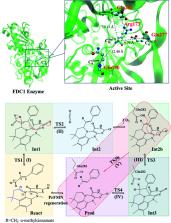
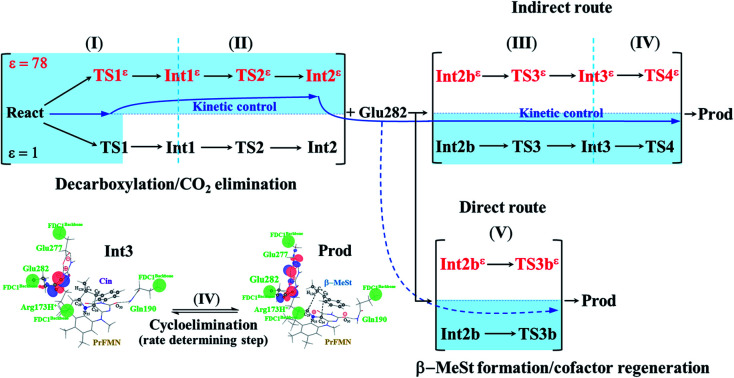
Enzymatic decarboxylation of α,β-unsaturated acid through ferulic acid decarboxylase (FDC1) has been of interest because this reaction has been anticipated to be a promising, environmentally friendly industrial process for producing styrene and its derivatives from natural resources. Because the local dielectric constant at the active site is not exactly known, enzymatic decarboxylation to generate β-methylstyrene (β-MeSt) was studied under two extreme conditions ( ε = 1 and 78 in the gas phase and aqueous solution, respectively) using the B3LYP/DZP method and transition state theory (TST). The model molecular clusters consisted of an α-methylcinnamate (Cin) substrate, a prenylated flavin mononucleotide (PrFMN) cofactor and all relevant residues of FDC1. Analysis of the equilibrium structures showed that the FDC1 backbone does not play the most important role in the decarboxylation process. The potential energy profiles confirmed that the increase in the polarity of the solvent could lead to significant changes in the energy barriers, especially for the transition states that involve proton transfer. Analysis of the rate constants confirmed the low/no quantum mechanical tunneling effect in the studied temperature range and that inclusion of the fluctuation of the local dielectric environment in the mechanistic model was essential. Because the computed rate constants are not compatible with the time resolution of the stopped-flow spectrophotometric experiment, the direct route for generating β-MeSt after CO 2 elimination (acid catalyst (2)) is unlikely to be utilized, thereby confirming that indirect cycloelimination in a low local dielectric environment is the rate determining step. The thermodynamic results showed that the elementary reactions that involve charge (proton) transfer are affected by solvent polarity, thereby leading to the conclusion that overall, the enzymatic decarboxylation of α,β-unsaturated acid is thermodynamically controlled at high ε. The entropy changes due to the generation of molecules in the active site appeared more pronounced than that due to only covalent bond breaking/formation or structural reorientation. This work examined in detail for the first time the scenarios in each elementary reaction and provided insight into the effect of the fluctuations in the local dielectric environment on the enzymatic decarboxylation of α,β-unsaturated acids. These results could be used as guidelines for further theoretical and experimental studies on the same and similar systems.
Abstract
The kinetically controlled path for enzymatic decarboxylation of α,β-unsaturated acid is proposed based on DFT and TST methods. The mechanism involves fluctuation of the local dielectric environment in the active site of the FDC1 enzyme.
Related collections
Most cited references1
- Record: found
- Abstract: not found
- Book: not found
Principles of chemical kinetics
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.