- Record: found
- Abstract: found
- Article: not found
Diagnostic Approaches For COVID-19: Lessons Learned and the Path Forward

Read this article at
Abstract
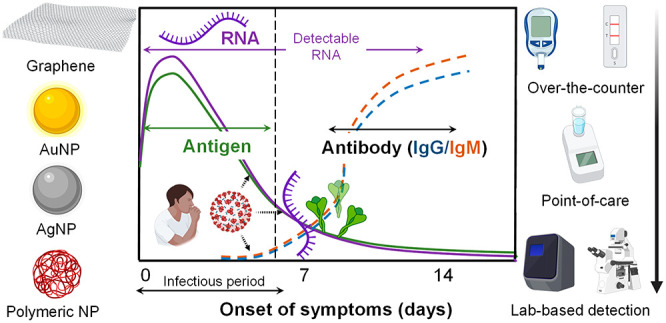
Coronavirus disease 2019 (COVID-19) is a transmitted respiratory disease caused by the infection of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although humankind has experienced several outbreaks of infectious diseases, the COVID-19 pandemic has the highest rate of infection and has had high levels of social and economic repercussions. The current COVID-19 pandemic has highlighted the limitations of existing virological tests, which have failed to be adopted at a rate to properly slow the rapid spread of SARS-CoV-2. Pandemic preparedness has developed as a focus of many governments around the world in the event of a future outbreak. Despite the largely widespread availability of vaccines, the importance of testing has not diminished to monitor the evolution of the virus and the resulting stages of the pandemic. Therefore, developing diagnostic technology that serves as a line of defense has become imperative. In particular, that test should satisfy three criteria to be widely adopted: simplicity, economic feasibility, and accessibility. At the heart of it all, it must enable early diagnosis in the course of infection to reduce spread. However, diagnostic manufacturers need guidance on the optimal characteristics of a virological test to ensure pandemic preparedness and to aid in the effective treatment of viral infections. Nanomaterials are a decisive element in developing COVID-19 diagnostic kits as well as a key contributor to enhance the performance of existing tests. Our objective is to develop a profile of the criteria that should be available in a platform as the target product. In this work, virus detection tests were evaluated from the perspective of the COVID-19 pandemic, and then we generalized the requirements to develop a target product profile for a platform for virus detection.
Related collections
Most cited references244
- Record: found
- Abstract: found
- Article: not found
Virological assessment of hospitalized patients with COVID-2019
- Record: found
- Abstract: found
- Article: found
Detection of SARS-CoV-2 in Different Types of Clinical Specimens
- Record: found
- Abstract: found
- Article: not found
