- Record: found
- Abstract: found
- Article: found
Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice
Read this article at
Abstract
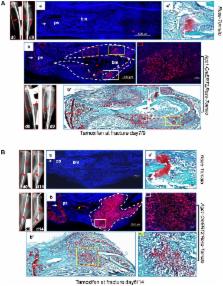
One of the crucial steps in endochondral bone formation is the replacement of a cartilage matrix produced by chondrocytes with bone trabeculae made by osteoblasts. However, the precise sources of osteoblasts responsible for trabecular bone formation have not been fully defined. To investigate whether cells derived from hypertrophic chondrocytes contribute to the osteoblast pool in trabecular bones, we genetically labeled either hypertrophic chondrocytes by Col10a1-Cre or chondrocytes by tamoxifen-induced Agc1-CreERT2 using EGFP, LacZ or Tomato expression. Both Cre drivers were specifically active in chondrocytic cells and not in perichondrium, in periosteum or in any of the osteoblast lineage cells. These in vivo experiments allowed us to follow the fate of cells labeled in Col10a1-Cre or Agc1-CreERT2 -expressing chondrocytes. After the labeling of chondrocytes, both during prenatal development and after birth, abundant labeled non-chondrocytic cells were present in the primary spongiosa. These cells were distributed throughout trabeculae surfaces and later were present in the endosteum, and embedded within the bone matrix. Co-expression studies using osteoblast markers indicated that a proportion of the non-chondrocytic cells derived from chondrocytes labeled by Col10a1-Cre or by Agc1-CreERT2 were functional osteoblasts. Hence, our results show that both chondrocytes prior to initial ossification and growth plate chondrocytes before or after birth have the capacity to undergo transdifferentiation to become osteoblasts. The osteoblasts derived from Col10a1-expressing hypertrophic chondrocytes represent about sixty percent of all mature osteoblasts in endochondral bones of one month old mice. A similar process of chondrocyte to osteoblast transdifferentiation was involved during bone fracture healing in adult mice. Thus, in addition to cells in the periosteum chondrocytes represent a major source of osteoblasts contributing to endochondral bone formation in vivo.
Author Summary
During endochondral bone formation, which is responsible for the generation of most bones in mammals and many other species, osteoblasts deposit a bone-specific matrix on the surface of cartilage scaffolds made by chondrocytes and hypertrophic chondrocytes. It has long been thought that the terminally differentiated chondrocytes in this cartilage scaffold undergo cell death. Here we demonstrate that chondrocytes can transdifferentiate into osteoblasts and that these transdifferentiated osteoblasts represent a substantial fraction of the bone forming cells in mice, We also provide evidence that chondrocytes can transdifferentiate into osteoblasts during bone fracture repair, a process similar to endochondral bone formation.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis.
- Record: found
- Abstract: found
- Article: not found
Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels.
- Record: found
- Abstract: found
- Article: not found