- Record: found
- Abstract: found
- Article: found
Positioning centrioles and centrosomes
Read this article at
Abstract
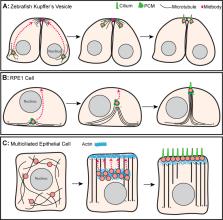
Hannaford and Rusan summarize the direct and indirect transport mechanisms by which centrosomes and centrioles are positioned in cells.
Abstract
Centrosomes are the primary microtubule organizer in eukaryotic cells. In addition to shaping the intracellular microtubule network and the mitotic spindle, centrosomes are responsible for positioning cilia and flagella. To fulfill these diverse functions, centrosomes must be properly located within cells, which requires that they undergo intracellular transport. Importantly, centrosome mispositioning has been linked to ciliopathies, cancer, and infertility. The mechanisms by which centrosomes migrate are diverse and context dependent. In many cells, centrosomes move via indirect motor transport, whereby centrosomal microtubules engage anchored motor proteins that exert forces on those microtubules, resulting in centrosome movement. However, in some cases, centrosomes move via direct motor transport, whereby the centrosome or centriole functions as cargo that directly binds molecular motors which then walk on stationary microtubules. In this review, we summarize the mechanisms of centrosome motility and the consequences of centrosome mispositioning and identify key questions that remain to be addressed.
Related collections
Most cited references167
- Record: found
- Abstract: found
- Article: not found
Genes and molecular pathways underpinning ciliopathies
- Record: found
- Abstract: found
- Article: not found
The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks.
- Record: found
- Abstract: found
- Article: not found