- Record: found
- Abstract: found
- Article: not found
Adult- and late-onset male hypogonadism: the clinical practice guidelines of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE)
Read this article at
Abstract
Purpose
To provide the evidence-based recommendations on the role of testosterone (T) on age-related symptoms and signs remains.
Methods
The Italian Society of Andrology and Sexual Medicine (SIAMS) and the and the Italian Society of Endocrinology (SIE) commissioned an expert task force to provide an updated guideline on adult-onset male hypogonadism. Derived recommendations were based on Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system.
Results
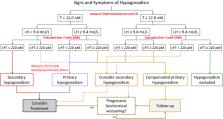
Clinical diagnosis of adult-onset hypogonadism should be based on a combination of clinical and biochemical parameters. Testosterone replacement therapy (TRT) should be offered to all symptomatic subjects with hypogonadism after the exclusion of possible contraindications. T gels and the long-acting injectable T are currently available preparations showing the best efficacy/safety profile. TRT can improve all aspects of sexual function, although its effect is limited in more complicated patients. Body composition (reducing fat mass and increasing lean mass) is improved after TRT, either in subjects with or without metabolic syndrome or type 2 diabetes. Conversely, the role of TRT in improving glycometabolic control is more conflicting. TRT can result in increasing bone mineral density, particularly at lumbar site, but no information on fracture risk is available. Limited data support the use of TRT for improving other outcomes, including mood frailty and mobility.
Conclusions
TRT can improve sexual function and body composition particularly in less complicated adult and in aging subjects with hypogonadism. When hypogonadism is adequately diagnosed, T appropriately prescribed and subjects correctly followed up, no short-term increased risk of adverse events is observed. Longer and larger studies are advisable to better clarify TRT long-term efficacy/safety profile.
Related collections
Most cited references173
- Record: found
- Abstract: found
- Article: not found
EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent
- Record: found
- Abstract: found
- Article: not found
Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline
- Record: found
- Abstract: found
- Article: not found
