- Record: found
- Abstract: found
- Article: found
TTK regulates proliferation and apoptosis of gastric cancer cells through the Akt‐mTOR pathway

Read this article at
Abstract
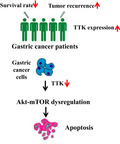
TTK (also known as Mps1) is the core component of the spindle assembly checkpoint, which ensures proper distribution of chromosomes to daughter cells to maintain genome integrity and to balance growth and division. However, the function of TTK in tumorigenesis has not been extensively studied, especially in relation to the development of gastric cancer. In this study, survival and tumor recurrence data related to TTK expression level in gastric cancer patients were collected and analyzed. We observed that TTK expression was negatively correlated with survival and tumor recurrence in vivo. TTK was also upregulated in gastric cancer cells and was observed to be essential for the proliferation and survival of gastric cancer cells. Knockdown of TTK inhibited proliferation and increased apoptosis. Furthermore, we report that TTK regulates the proliferation and apoptosis of tumor cells through the Akt‐mTOR pathway. Knockdown of TTK inhibited activation of Akt‐mTOR signaling. In summary, our data indicate that TTK is involved in the regulation of gastric cancer proliferation and apoptosis.
Abstract
We report here that high TTK expression is correlated with lower survival rate and higher tumor recurrence in gastric cancer patients. Knockdown or inhibition of TTK in gastric cancer cells resulted in Akt‐mTOR dysregulation and enhanced apoptosis of cancer cells. Our data suggest that TTK is involved in the regulation of gastric cancer proliferation and apoptosis.
Related collections
Most cited references20

- Record: found
- Abstract: found
- Article: found
Kinase-targeted cancer therapies: progress, challenges and future directions

- Record: found
- Abstract: found
- Article: found
The Clinical Evidence Linking Helicobacter pylori to Gastric Cancer
- Record: found
- Abstract: found
- Article: not found