- Record: found
- Abstract: found
- Article: found
Efficacy of adalimumab across subgroups of patients with moderate-to-severe chronic plaque psoriasis of the hands and/or feet: post hoc analysis of REACH
Read this article at
Abstract
Background
The Randomized Controlled Evaluation of Adalimumab in Treatment of Chronic Plaque Psoriasis of the Hands and Feet (REACH) trial demonstrated that adalimumab was efficacious and well-tolerated for the treatment of hand and/or foot psoriasis through 28 weeks.
Objective
To evaluate the effects of patient baseline characteristics on efficacy of adalimumab treatment of hand and/or foot psoriasis.
Methods
Patients with moderate-to-severe chronic plaque psoriasis of the hands and/or feet were randomized 2: 1 to adalimumab or placebo during the 16 week, double-blind period of REACH. Primary endpoint was percentage of patients achieving Physician’s Global Assessment of the hands and/or feet of clear/almost clear at week 16. Post hoc analyses evaluated effects of baseline patient characteristics on the primary endpoint. Patients with nail psoriasis at baseline were assessed for association of Nail Psoriasis Severity Index (NAPSI) 50 response with efficacy outcomes at week 16.
Results
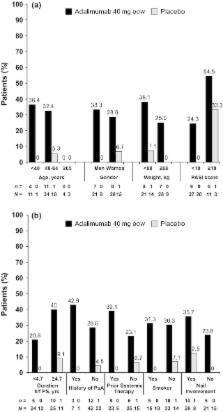
Seventy-two patients (49 adalimumab: 23 placebo) were analysed. Greater percentages of adalimumab-treated patients achieved the primary endpoint vs. placebo across all subgroups. Among 31 patients with nail psoriasis, a greater percentage of adalimumab-treated patients achieved NAPSI 50 (56.5%) vs. placebo (12.5%) at week 16. In adalimumab-treated patients, greater percentages of NAPSI 50 Responders vs. Non-responders achieved the primary endpoint, and had greater improvements in erythema, scaling, induration and fissuring, Dermatology Life Quality Index, and pain scores.
Conclusions
Adalimumab was efficacious in treating chronic plaque psoriasis of the hands and/or feet over 16 weeks, regardless of baseline characteristics. Marked improvement in nail psoriasis among adalimumab-treated patients correlated with significant improvements in skin disease and patient-reported outcomes.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Psoriasis of the nail: anatomy, pathology, clinical presentation, and a review of the literature on therapy.
- Record: found
- Abstract: not found
- Article: not found
Treatment of psoriasis.
- Record: found
- Abstract: found
- Article: not found