- Record: found
- Abstract: found
- Article: found
Ibrutinib treatment inhibits breast cancer progression and metastasis by inducing conversion of myeloid-derived suppressor cells to dendritic cells
Read this article at
Abstract
Background
Ibrutinib is a Bruton’s tyrosine kinase (BTK) and interleukin-2-inducible kinase (ITK) inhibitor used for treating chronic lymphocytic leukaemia (CLL) and other cancers. Although ibrutinib is known to inhibit the growth of breast cancer cell growth in vitro, its impact on the treatment and metastasis of breast cancer is unclear.
Methods
Using an orthotopic mouse breast cancer model, we show that ibrutinib inhibits the progression and metastasis of breast cancer.
Results
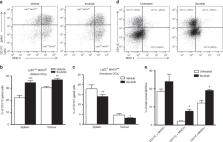
Ibrutinib inhibited proliferation of cancer cells in vitro, and Ibrutinib-treated mice displayed significantly lower tumour burdens and metastasis compared to controls. Furthermore, the spleens and tumours from Ibrutinib-treated mice contained more mature DCs and lower numbers of myeloid-derived suppressor cells (MDSCs), which promote disease progression and are linked to poor prognosis. We also confirmed that ex vivo treatment of MDSCs with ibrutinib switched their phenotype to mature DCs and significantly enhanced MHCII expression. Further, ibrutinib treatment promoted T cell proliferation and effector functions leading to the induction of antitumour T H1 and CTL immune responses.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Oncology meets immunology: the cancer-immunity cycle.
- Record: found
- Abstract: found
- Article: not found
Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion.
- Record: found
- Abstract: found
- Article: not found