- Record: found
- Abstract: found
- Article: not found
Crystal structures of Ryanodine Receptor SPRY1 and tandem-repeat domains reveal a critical FKBP12 binding determinant
Read this article at
Abstract
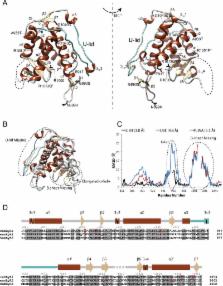
Ryanodine receptors (RyRs) form calcium release channels located in the membranes of the sarcoplasmic and endoplasmic reticulum. RyRs play a major role in excitation-contraction coupling and other Ca 2+-dependent signaling events, and consist of several globular domains that together form a large assembly. Here we describe the crystal structures of the SPRY1 and tandem-repeat domains at 1.2 – 1.5Å resolution, which reveal several structural elements not detected in recent cryo-EM reconstructions of RyRs. The cryo-EM studies disagree on the position of SPRY domains, which had been proposed based on homology modeling. Computational docking of the crystal structures, combined with FRET studies, show that the SPRY1 domain is located next to FK506-Binding Protein (FKBP). Molecular dynamics flexible fitting and mutagenesis experiments suggest a hydrophobic cluster within SPRY1 that is crucial for FKBP binding. A RyR1 disease mutation, N760D, appears to directly impact FKBP binding through interfering with SPRY1 folding.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics.
- Record: found
- Abstract: found
- Article: not found
Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging.
- Record: found
- Abstract: found
- Article: not found
