- Record: found
- Abstract: found
- Article: found
T-cell redirecting bispecific and trispecific antibodies in multiple myeloma beyond BCMA
Read this article at
Abstract
Purpose of review
B-cell maturation antigen (BCMA)-directed T-cell immunotherapies, such as chimeric antigen receptor T-cells (CAR T-cells) and bispecific antibodies (BsAbs) have markedly improved the survival of triple-class refractory multiple myeloma (MM). However, the majority of patients still develops disease progression, underlining the need for new agents for these patients.
Recent findings
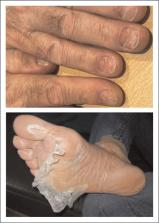
Novel T-cell redirecting BsAbs targeting alternative tumor-associated antigens have shown great promise in heavily pretreated MM, including patients previously exposed to BCMA-directed therapies. This includes the G-protein-coupled receptor class 5 member D (GPRC5D)-targeting BsAbs talquetamab and forimtamig, as well as the Fc receptor-homolog 5 (FcRH5)-targeting BsAb cevostamab. Toxicity associated with these BsAbs includes cytokine-release syndrome, cytopenias, and infections. In addition, GPRC5D-targeting BsAbs are associated with specific ‘on target/off tumor’ toxicities including rash, nail disorders, and dysgeusia. Trispecifc antibodies targeting two different MM-associated antigens to prevent antigen escape are in early clinical development, as well as trispecific antibodies (TsAbs) that provide an additional co-stimulatory signal to T-cells to prevent their exhaustion.
Summary
Various T-cell redirecting BsAbs are in advanced stages of clinical development with promising activity and a manageable toxicity profile. Ongoing studies are evaluating combination strategies, fixed-duration treatment, and use of BsAbs in earlier lines of therapy. TsAbs hold great promise for the future.
Related collections
Most cited references82
- Record: found
- Abstract: not found
- Article: not found
Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma
- Record: found
- Abstract: not found
- Article: not found
Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study
- Record: found
- Abstract: found
- Article: not found