- Record: found
- Abstract: found
- Article: found
Interleukin-1β, Oxidative Stress, and Abnormal Calcium Handling Mediate Diabetic Arrhythmic Risk

Read this article at
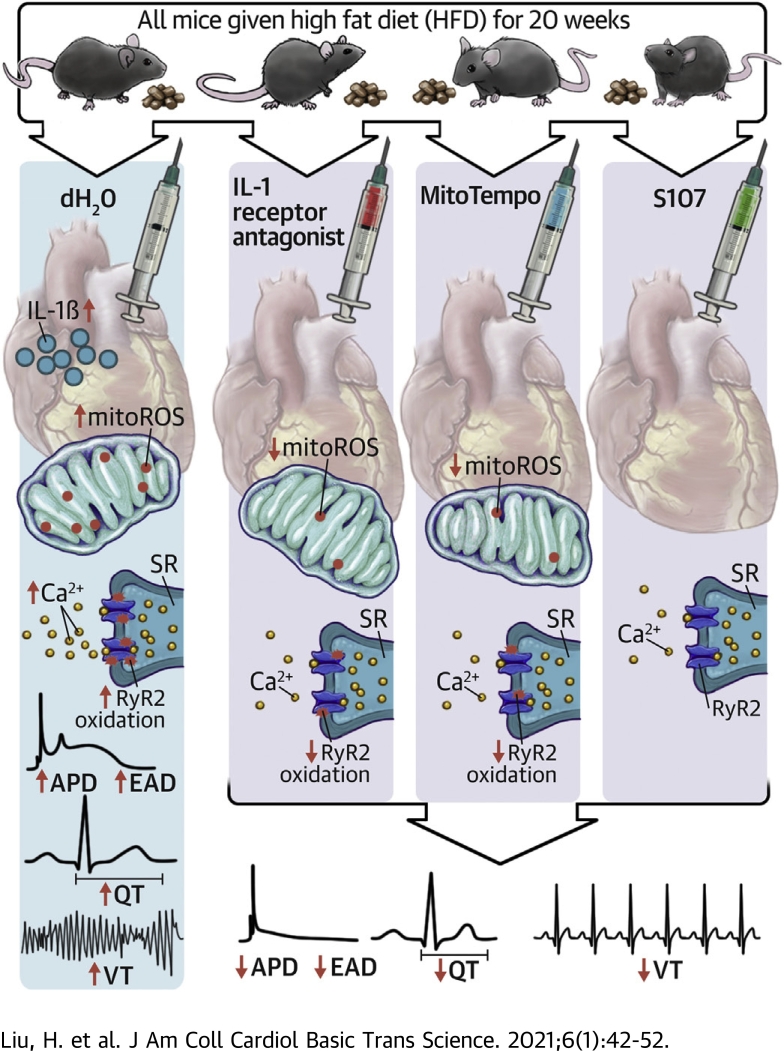
Visual Abstract
Highlights
-
•
Diabetes-induced arrhythmic risk involved activation of innate immunity, elevation of IL-1β, mitochondrial oxidative stress, SR calcium release channel oxidation, and QT prolongation.
-
•
Diabetes-induced arrhythmic risk could be inhibited by IL-1β antagonism, mitoROS scavenging, and SR calcium release stabilization.
-
•
The relationship of inflammation and arrhythmic risk may account for increased susceptibility of diabetic patients to the effects of COVID-19.
Summary
Diabetes mellitus (DM) is associated with increased arrhythmia. Type 2 DM (T2DM) mice showed prolonged QT interval and increased ventricular arrhythmic inducibility, accompanied by elevated cardiac interleukin (IL)-1β, increased mitochondrial reactive oxygen species (mitoROS), and oxidation of the sarcoplasmic reticulum (SR) Ca 2+ release channel (ryanodine receptor 2 [RyR2]). Inhibiting IL-1β and mitoROS reduced RyR2 oxidation and the ventricular arrhythmia in DM. Inhibiting SR Ca2 + leak by stabilizing the oxidized RyR2 channel reversed the diabetic arrhythmic risk. In conclusion, cardiac IL-1β mediated the DM-associated arrhythmia through mitoROS generation that enhances SR Ca 2+ leak. The mechanistic link between inflammation and arrhythmias provides new therapeutic options.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease.
- Record: found
- Abstract: found
- Article: not found
Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector?
- Record: found
- Abstract: found
- Article: not found