- Record: found
- Abstract: found
- Article: found
Targeting cGAS/STING signaling-mediated myeloid immune cell dysfunction in TIME
Read this article at
Abstract
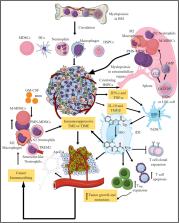
Myeloid immune cells (MICs) are potent innate immune cells serving as first responders to invading pathogens and internal changes to cellular homeostasis. Cancer is a stage of altered cellular homeostasis that can originate in response to different pathogens, chemical carcinogens, and internal genetic/epigenetic changes. MICs express several pattern recognition receptors (PRRs) on their membranes, cytosol, and organelles, recognizing systemic, tissue, and organ-specific altered homeostasis. cGAS/STING signaling is a cytosolic PRR system for identifying cytosolic double-stranded DNA (dsDNA) in a sequence-independent but size-dependent manner. The longer the cytosolic dsDNA size, the stronger the cGAS/STING signaling activation with increased type 1 interferon (IFN) and NF-κB-dependent cytokines and chemokines’ generation. The present article discusses tumor-supportive changes occurring in the tumor microenvironment (TME) or tumor immune microenvironment (TIME) MICs, specifically emphasizing cGAS/STING signaling-dependent alteration. The article further discusses utilizing MIC-specific cGAS/STING signaling modulation as critical tumor immunotherapy to alter TIME.
Related collections
Most cited references236
- Record: found
- Abstract: found
- Article: not found
Inflammation and cancer.
- Record: found
- Abstract: found
- Article: not found
Macrophage plasticity and polarization: in vivo veritas.
- Record: found
- Abstract: found
- Article: not found