- Record: found
- Abstract: found
- Article: found
Disproportionation of Co 2+ in the Topochemically Reduced Oxide LaSrCoRuO 5
Read this article at
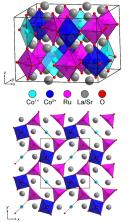
Abstract
Complex transition‐metal oxides exhibit a wide variety of chemical and physical properties which are a strong function the local electronic states of the transition‐metal centres, as determined by a combination of metal oxidation state and local coordination environment. Topochemical reduction of the double perovskite oxide, LaSrCoRuO 6, using Zr, yields LaSrCoRuO 5. This reduced phase contains an ordered array of apex‐linked square‐based pyramidal Ru 3+O 5, square‐planar Co 1+O 4 and octahedral Co 3+O 6 units, consistent with the coordination‐geometry driven disproportionation of Co 2+. Coordination‐geometry driven disproportionation of d 7 transition‐metal cations (e.g. Rh 2+, Pd 3+, Pt 3+) is common in complex oxides containing 4d and 5d metals. However, the weak ligand field experienced by a 3d transition‐metal such as cobalt leads to the expectation that d 7+ Co 2+ should be stable to disproportionation in oxide environments, so the presence of Co 1+O 4 and Co 3+O 6 units in LaSrCoRuO 5 is surprising. Low‐temperature measurements indicate LaSrCoRuO 5 adopts a ferromagnetically ordered state below 120 K due to couplings between S= 1/ 2 Ru 3+ and S=1 Co 1+.
Abstract
Related collections
Most cited references1
- Record: found
- Abstract: not found
- Book: not found
