- Record: found
- Abstract: found
- Article: found
Escherichia coli BdcA controls biofilm dispersal in Pseudomonas aeruginosa and Rhizobium meliloti
Read this article at
Abstract
Background
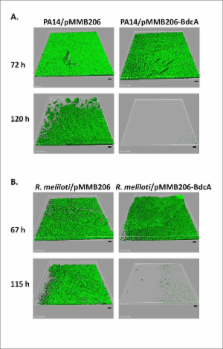
Previously we showed that BdcA controls Escherichia coli biofilm dispersal by binding the ubiquitous bacterial signal cyclic diguanylate (c-di-GMP); upon reducing the concentration of c-di-GMP, the cell shifts to the planktonic state by increasing motility, decreasing aggregation, and decreasing production of biofilm adhesins.
Findings
Here we report that BdcA also increases biofilm dispersal in other Gram-negative bacteria including Pseudomonas aeruginosa, Pseudomonas fluorescens, and Rhizobium meliloti. BdcA binds c-di-GMP in these strains and thereby reduces the effective c-di-GMP concentrations as demonstrated by increases in swimming motility and swarming motility as well as by a reduction in extracellular polysaccharide production. We also develop a method to displace existing biofilms by adding BdcA via conjugation from E. coli in mixed-species biofilms.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Lung infections associated with cystic fibrosis.
- Record: found
- Abstract: found
- Article: not found
An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants.
- Record: found
- Abstract: found
- Article: not found