- Record: found
- Abstract: found
- Article: found
Amelioration of Aspirin Induced Oxidative Impairment and Apoptotic Cell Death by a Novel Antioxidant Protein Molecule Isolated from the Herb Phyllanthus niruri
Read this article at
Abstract
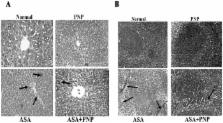
Aspirin has been used for a long time as an analgesic and anti-pyretic drug. Limitations of its use, however, remain for the gastro-intestinal side effects and erosions. Although the role of aspirin on gastro-intestinal injury has been extensively studied, the molecular mechanisms underlying aspirin -induced liver and spleen pathophysiology are poorly defined. The present study has been conducted to investigate whether phyllanthus niruri protein (PNP) possesses any protective role against aspirin mediated liver and spleen tissue toxicity, and if so, what signaling pathways it utilizes to convey its protective action. Aspirin administration in mice enhanced serum marker (ALP) levels, reactive oxygen species (ROS) generation, reduced antioxidant power and altered oxidative stress related biochemical parameters in liver and spleen tissues. Moreover, we observed that aspirin intoxication activated both the extrinsic and intrinsic apoptotic pathways, as well as down regulated NF-κB activation and the phosphorylation of p38 and JNK MAPKs. Histological assessments and TUNEL assay also supported that aspirin induced tissue damages are apoptotic in nature. PNP treatment after aspirin exposure effectively neutralizes all these abnormalities via the activation of survival PI3k/Akt pathways. Combining all results suggest that PNP could be a potential protective agent to protect liver and spleen from the detrimental effects of aspirin.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta.
- Record: found
- Abstract: found
- Article: not found