- Record: found
- Abstract: found
- Article: not found
The Functions of IL-23 and IL-2 on Driving Autoimmune Effector T-helper 17 Cells into the Memory Pool in Dry Eye Disease
Read this article at
Abstract
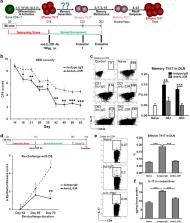
Long-lived memory Th17 cells actively mediate the chronic inflammation in autoimmune disorders, including dry eye disease (DED). The mechanisms responsible for the maintenance and reactivation of these cells in autoimmunity have been subject of investigation. However, the process through which memory Th17 are generated from their effector precursors remains to be elucidated. Herein, using our murine model of DED, we detect a linear transition from effector to memory Th17 cells during the abatement phase of acute inflammation, which is accompanied by persistently high levels of IL-23 and diminished levels of IL-2. Additionally, in vitro culture of effector Th17 cells derived from the DED animals with IL-23, but not IL-2, leads to significant generation of memory Th17 cells, along with up-regulated expressions of IL-7R and IL-15R by these cells. Furthermore, supplementation of IL-2 abolishes, and blockade of IL-2 enhances IL-23-induced generation of memory Th17 cells in vitro. Finally, in vivo blockade of IL-23 signaling during the contraction phase of primary response inhibits the generation of memory Th17 cells from their effector precursors. Together, our data demonstrate a new dichotomy between IL-23 and IL-2 in driving effector Th17 cells into the memory pool in autoimmune-mediated ocular surface inflammation.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Generation of Pathogenic Th17 Cells in the Absence of TGF-β Signaling
- Record: found
- Abstract: found
- Article: not found
The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo.
- Record: found
- Abstract: found
- Article: not found
