- Record: found
- Abstract: found
- Article: found
Resveratrol Mediated Modulation of Sirt-1/Runx2 Promotes Osteogenic Differentiation of Mesenchymal Stem Cells: Potential Role of Runx2 Deacetylation
Read this article at
Abstract
Objective
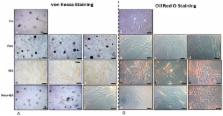
Osteogenic repair in response to bone injury is characterized by activation and differentiation of mesenchymal stem cells (MSCs) to osteoblasts. This study determined whether activation of Sirt-1 (a NAD +-dependent histone deacetylase) by the phytoestrogen resveratrol affects osteogenic differentiation.
Methods
Monolayer and high-density cultures of MSCs and pre-osteoblastic cells were treated with an osteogenic induction medium with/without the Sirt-1 inhibitor nicotinamide or/and resveratrol in a concentration dependent manner.
Results
MSCs and pre-osteoblastic cells differentiated to osteoblasts when exposed to osteogenic-induction medium. The osteogenic response was blocked by nicotinamide, resulting in adipogenic differentiation and expression of the adipose transcription regulator PPAR-γ (peroxisome proliferator-activated receptor). However, in nicotinamide-treated cultures, pre-treatment with resveratrol significantly enhanced osteogenesis by increasing expression of Runx2 (bone specific transcription factor) and decreasing expression of PPAR-γ. Activation of Sirt-1 by resveratrol in MSCs increased its binding to PPAR-γ and repressed PPAR-γ activity by involving its cofactor NCoR (nuclear receptor co-repressor). The modulatory effects of resveratrol on nicotinamide-induced expression of PPAR-γ and its cofactor NCoR were found to be mediated, at least in part, by Sirt-1/Runx2 association and deacetylation of Runx2.
Finally, knockdown of Sirt-1 by using antisense oligonucleotides downregulated the expression of Sirt-1 protein and abolished the inhibitory effects of resveratrol, namely nicotinamide-induced Sirt-1 suppression and Runx2 acetylation, suggesting that the acetylated content of Runx2 is related to downregulated Sirt-1 expression.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction.
- Record: found
- Abstract: found
- Article: not found
Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain.
- Record: found
- Abstract: found
- Article: not found