- Record: found
- Abstract: found
- Article: found
Barriers to COVID-19 Health Products in Low-and Middle-Income Countries During the COVID-19 Pandemic: A Rapid Systematic Review and Evidence Synthesis
Read this article at
Abstract
Introduction
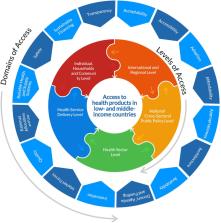
The coronavirus disease 2019 (COVID-19) pandemic has intensified the urgency in addressing pressing global health access challenges and has also laid bare the pervasive structural and systemic inequities that make certain segments of society more vulnerable to the tragic consequences of the disease. This rapid systematic review analyses the barriers to COVID-19 health products in low-and middle-income countries (LMICs). It does so from the canon of global health equity and access to medicines by proposing an access to health products in low-and middle-income countries framework and typology adapted to underscore the complex interactive and multiplicative nature and effects of barriers to health products and their root cause as they coexist across different levels of society in LMICs.
Methods
Modified versions of the Joanna Briggs Institute (JBI) reviewers' manual for evidence synthesis of systematic reviews and the PRISMA-ScR framework were used to guide the search strategy, identification, and screening of biomedical, social science, and gray literature published in English between 1 January 2020 and 30 April 2021.
Results
The initial search resulted in 5,956 articles, with 72 articles included in this review after screening protocol and inclusion criteria were applied. Thirty one percent of the articles focused on Africa. The review revealed that barriers to COVID-19 health products were commonly caused by market forces (64%), the unavailability (53%), inaccessibility (42%), and unaffordability (35%), of the products, incongruent donors' agenda and funding (33%) and unreliable health and supply systems (28%). They commonly existed at the international and regional (79%), health sectoral (46%), and national cross-sectoral [public policy] (19%) levels. The historical heritage of colonialism in LMICs was a commonly attributed root cause of the barriers to COVID-19 health products in developing countries.
Related collections
Most cited references128
- Record: found
- Abstract: not found
- Article: not found
Scoping studies: towards a methodological framework
- Record: found
- Abstract: found
- Article: not found