- Record: found
- Abstract: found
- Article: not found
Theoretical Study on the Epimerization of Azlactone Rings: Keto–Enol Tautomerism or Base-Mediated Racemization?

Read this article at
Abstract
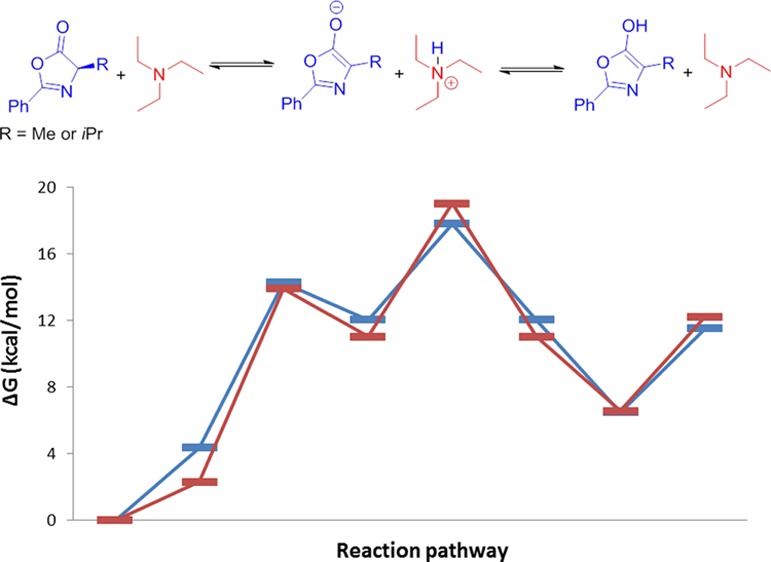
Azlactones are versatile heterocycles employed in a diversity of transformations; the main drawback of these cycles consists in the epimerization of the α-carbonyl stereocenter during its preparation. We hereby present a theoretical study to explain how the racemization occurs. Two hypotheses were investigated: the keto–enol tautomerism and the base-mediated racemization, through an enolate intermediate. The results showed that the latter is consistent with the experimental data and can spontaneously occur at room temperature. The same pathway was evaluated for 2-alcoxy azlactone, showing a slower epimerization ratio, consistent with the literature data.
Related collections
Most cited references38
- Record: found
- Abstract: not found
- Article: not found
Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations
- Record: found
- Abstract: not found
- Article: not found
Highly Efficient Dynamic Kinetic Resolution of Azlactones by Urea-Based Bifunctional Organocatalysts
- Record: found
- Abstract: found
- Article: not found
