- Record: found
- Abstract: found
- Article: found
Associations between Fatty Acid-Binding Protein 4–A Proinflammatory Adipokine and Insulin Resistance, Gestational and Type 2 Diabetes Mellitus
Read this article at
Abstract
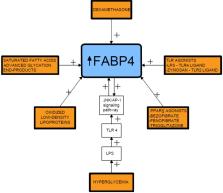
There is ample scientific evidence to suggest a link between the fatty acid-binding protein 4 (FABP4) and insulin resistance, gestational (GDM), and type 2 (T2DM) diabetes mellitus. This novel proinflammatory adipokine is engaged in the regulation of lipid metabolism at the cellular level. The molecule takes part in lipid oxidation, the regulation of transcription as well as the synthesis of membranes. An involvement of FABP4 in the pathogenesis of obesity and insulin resistance seems to be mediated via FABP4-dependent peroxisome proliferator-activated receptor γ (PPARγ) inhibition. A considerable number of studies have shown that plasma concentrations of FABP4 is increased in obesity and T2DM, and that circulating FABP4 levels are correlated with certain clinical parameters, such as body mass index, insulin resistance, and dyslipidemia. Since plasma-circulating FABP4 has the potential to modulate the function of several types of cells, it appears to be of extreme interest to try to develop potential therapeutic strategies targeting the pathogenesis of metabolic diseases in this respect. In this manuscript, representing a detailed review of the literature on FABP4 and the abovementioned metabolic disorders, various mechanisms of the interaction of FABP4 with insulin signaling pathways are thoroughly discussed. Clinical aspects of insulin resistance in diabetic patients, including women diagnosed with GDM, are analyzed as well.
Related collections
Most cited references83
- Record: found
- Abstract: found
- Article: not found
Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance.
- Record: found
- Abstract: found
- Article: not found
A central role for inflammation in the pathogenesis of diabetic retinopathy.
- Record: found
- Abstract: found
- Article: not found