- Record: found
- Abstract: found
- Article: found
Real-time sensing of MAPK signaling in medulloblastoma cells reveals cellular evasion mechanism counteracting dasatinib blockade of ERK activation during invasion
Read this article at
Highlights
-
•
Growth factor signaling causes sustained nuclear ERK1/2 activation.
-
•
The SCR and BCR/ABL inhibitor dasatinib blocks ERK1/2 and represses cell invasion.
-
•
EGF-stimulated cells may escape dasatinib inhibition of invasion through mesenchymal to amoeboid transition.
-
•
Combined inhibition of SRC and Rho-kinase signaling is necessary to completely block EGF-induced invasion.
Abstract
Aberrantly activated kinase signaling pathways drive invasion and dissemination in medulloblastoma (MB). A majority of tumor-promoting kinase signaling pathways feed into the mitogen-activated protein kinase (MAPK) extracellular regulated kinase (ERK1/2) pathway. The activation status of ERK1/2 during invasion of MB cells is not known and its implication in invasion control unclear.
We established a synthetic kinase activation relocation sensor (SKARS) for the MAPK ERK1/2 pathway in MB cells for real-time measuring of drug response. We used 3D invasion assays and organotypic cerebellum slice culture to test drug effects in a physiologically relevant tissue environment.
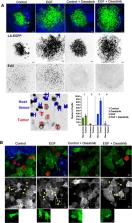
We found that hepatocyte growth factor (HGF), epidermal growth factor (EGF), or basic fibroblast growth factor (bFGF) caused rapid nuclear ERK1/2 activation in MB cells, which persisted for several hours. Concomitant treatment with the BCR/ABL kinase inhibitor dasatinib completely repressed nuclear ERK1/2 activity induced by HGF and EGF but not by bFGF. Increased nuclear ERK1/2 activity correlated positively with speed of invasion. Dasatinib blocked ERK-associated invasion in the majority of cells, but we also observed fast-invading cells with low ERK1/2 activity. These ERK1/2-low, fast-moving cells displayed a rounded morphology, while ERK-high fast-moving cells displayed a mesenchymal morphology. Dasatinib effectively blocked EGF-induced proliferation while it only moderately repressed tissue invasion, indicating that a subset of cells may evade invasion repression by dasatinib through non-mesenchymal motility. Thus, growth factor-induced nuclear activation of ERK1/2 is associated with mesenchymal motility and proliferation in MB cells and can be blocked with the BCR/ABL kinase inhibitor dasatinib.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Every step of the way: integrins in cancer progression and metastasis
- Record: found
- Abstract: found
- Article: not found
Overriding imatinib resistance with a novel ABL kinase inhibitor.

- Record: found
- Abstract: found
- Article: found