- Record: found
- Abstract: found
- Article: found
COVID-19 vaccination intention in the UK: results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey
Read this article at
ABSTRACT
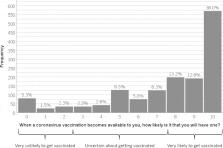
To investigate factors associated with intention to be vaccinated against COVID-19 we conducted a cross-sectional survey of 1,500 UK adults, recruited from an existing online research panel. Data were collected between 14th and 17th July 2020. We used linear regression analyses to investigate associations between intention to be vaccinated for COVID-19 “when a vaccine becomes available to you” and sociodemographic factors, previous influenza vaccination, general vaccine attitudes and beliefs, attitudes and beliefs about COVID-19, and attitudes and beliefs about a COVID-19 vaccination. 64% of participants reported being very likely to be vaccinated against COVID-19, 27% were unsure, and 9% reported being very unlikely to be vaccinated. Personal and clinical characteristics, previous influenza vaccination, general vaccination beliefs, and beliefs and attitudes about COVID-19 and a COVID-19 vaccination explained 76% of the variance in vaccination intention. Intention to be vaccinated was associated with more positive general COVID-19 vaccination beliefs and attitudes, weaker beliefs that the vaccination would cause side effects or be unsafe, greater perceived information sufficiency to make an informed decision about COVID-19 vaccination, greater perceived risk of COVID-19 to others (but not risk to oneself), older age, and having been vaccinated for influenza last winter (2019/20). Despite uncertainty around the details of a COVID-19 vaccination, most participants reported intending to be vaccinated for COVID-19. Actual uptake may be lower. Vaccination intention reflects general vaccine beliefs and attitudes. Campaigns and messaging about a COVID-19 vaccination could consider emphasizing the risk of COVID-19 to others and necessity for everyone to be vaccinated.
Related collections
Most cited references29

- Record: found
- Abstract: found
- Article: found