- Record: found
- Abstract: found
- Article: found
Transition from vehicle to Grotthuss proton transfer in a nanosized flask: cryogenic ion spectroscopy of protonated p-aminobenzoic acid solvated with D 2O†
Read this article at
Abstract
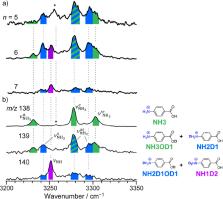
Proton transfer (PT) is one of the most ubiquitous reactions in chemistry and life science. The unique nature of PT has been rationalized not by the transport of a solvated proton (vehicle mechanism) but by the Grotthuss mechanism in which a proton is transported to the nearest proton acceptor along a hydrogen-bonded network. However, clear experimental evidence of the Grotthuss mechanism has not been reported yet. Herein we show by infrared spectroscopy that a vehicle-type PT occurs in the penta- and hexahydrated clusters of protonated p-aminobenzoic acid, while Grotthuss-type PT is observed in heptahydrated clusters, indicating a change in the PT mechanism depending on the degree of hydration. These findings emphasize the importance of the usually ignored vehicle mechanism as well as the degree of hydration. It highlights the possibility of controlling the PT mechanism by the number of water molecules in chemical and biological environments.
Abstract
Cryogenic double ion trap IR spectroscopy combined with isotopic labelling reveals that the solvent-mediated intracluster proton transfer mechanism in microhydrated protonated p-aminobenzoic acid changes from vehicle to Grotthuss between n = 5 and 7.

Related collections
Most cited references2
- Record: found
- Abstract: not found
- Article: not found
Diffusion-free Grotthuss topochemistry for high-rate and long-life proton batteries
- Record: found
- Abstract: found
- Article: not found