- Record: found
- Abstract: found
- Article: found
Melatonin inhibits proliferation, migration, and invasion by inducing ROS-mediated apoptosis via suppression of the PI3K/Akt/mTOR signaling pathway in gallbladder cancer cells
Read this article at
Abstract
Background: Melatonin is an indolic compound mainly secreted by the pineal gland and plays a vital role in the regulation of circadian rhythms and cancer therapy. However, the effects of melatonin in gallbladder cancer (GBC) and the related mechanism remain unknown.
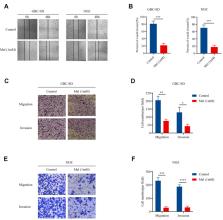
Methods: In this study, the antitumor activity of melatonin on gallbladder cancer was explored both in vitro and in vivo. After treatment with different concentrations of melatonin, the cell viability, migration, and invasion of gallbladder cancer cells (NOZ and GBC-SD cells) were evaluated by CCK-8 assay, wound healing, and Transwell assay.
Results: The results showed that melatonin inhibited growth, migration, and invasion of gallbladder cancer cells. Subsequently, the assays suggested that melatonin significantly induced apoptosis in gallbladder cancer cells and altered the expression of the apoptotic proteins, including Bax, Bcl-2, cytochrome C, cleaved caspase-3, and PARP. Besides, the intracellular reactive oxygen species (ROS) was found to be upregulated after melatonin treatment in gallbladder cancer cells. Melatonin was found to suppress the PI3K/Akt/mTOR signaling pathway in a time-dependent manner by inhibiting the phosphorylation of PI3K, Akt, and mTOR. Treatment with N-acetyl-L-cysteine (NAC) or 740 Y-P remarkably attenuated the antitumor effects of melatonin in NOZ and GBC-SD cells. Finally, melatonin suppressed the growth of GBC-SD cells in an athymic nude mice xenograft model in vivo.
Conclusions: Our study revealed that melatonin could induce apoptosis by suppressing the PI3K/Akt/mTOR signaling pathway. Therefore, melatonin might serve as a potential therapeutic drug in the future treatment of gallbladder cancer.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release.
- Record: found
- Abstract: found
- Article: not found
The PI3K Pathway in Human Disease.
- Record: found
- Abstract: found
- Article: not found