- Record: found
- Abstract: found
- Article: found
Recombinant Immunomodulating Lentogenic or Mesogenic Oncolytic Newcastle Disease Virus for Treatment of Pancreatic Adenocarcinoma
Read this article at
Abstract
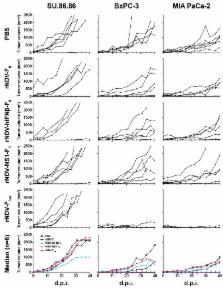
Oncolytic Newcastle disease virus (NDV) might be a promising new therapeutic agent for the treatment of pancreatic cancer. We evaluated recombinant NDVs (rNDVs) expressing interferon (rNDV-hIFNβ-F 0) or an IFN antagonistic protein (rNDV-NS1-F 0), as well as rNDV with increased virulence (rNDV-F 3aa) for oncolytic efficacy in human pancreatic adenocarcinoma cells. Expression of additional proteins did not hamper virus replication or cytotoxic effects on itself. However, expression of interferon, but not NS1, resulted in loss of multicycle replication. Conversely, increasing the virulence (rNDV-F 3aa) resulted in enhanced replication of the virus. Type I interferon was produced in high amounts by all tumor cells inoculated with rNDV-hIFNβ-F 0, while inoculation with rNDV-NS1-F 0 resulted in a complete block of interferon production in most cells. Inoculation of human pancreatic adenocarcinoma cells with rNDV-F 3aa caused markedly more cytotoxicity compared to rNDV-F 0, while inoculation with rNDV-hIFNβ-F 0 and rNDV-NS1-F 0 induced cytotoxic effects comparable to those induced by the parental rNDV-F 0. Evaluation in vivo using mice bearing subcutaneous pancreatic cancer xenografts revealed that only intratumoral injection with rNDV-F 3aa resulted in regression of tumors. We conclude that although lentogenic rNDVs harboring proteins that modulate the type I interferon pathway proteins do have an oncolytic effect, a more virulent mesogenic rNDV might be needed to improve oncolytic efficacy.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial.
- Record: found
- Abstract: found
- Article: not found
Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy.
- Record: found
- Abstract: found
- Article: not found