- Record: found
- Abstract: found
- Article: found
Simplified Multiple-Well Approach for the Master Equation Modeling of Blackbody Infrared Radiative Dissociation of Hydrated Carbonate Radical Anions
Read this article at
Abstract

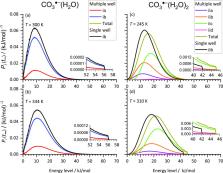
Blackbody infrared radiative dissociation (BIRD) in a collision-free environment is a powerful method for the experimental determination of bond dissociation energies. In this work, we investigate temperature-dependent BIRD of CO 3 ·–(H 2O) 1,2 at 250–330 K to determine water binding energies and assess the influence of multiple isomers on the dissociation kinetics. The ions are trapped in a Fourier-transform ion cyclotron resonance mass spectrometer, mass selected, and their BIRD kinetics are recorded at varying temperatures. Experimental BIRD rates as a function of temperature are fitted with rates obtained from master equation modeling (MEM), using the water binding energy as a fit parameter. MEM accounts for the absorption and emission of photons from black-body radiation, described with harmonic frequencies and infrared intensities from quantum chemical calculations. The dissociation rates as a function of internal energy are calculated by Rice–Ramsperger–Kassel–Marcus theory. Both single-well and multiple-well MEM approaches are used. Dissociation energies derived in this way from the experimental data are 56 ± 6 and 45 ± 3 kJ/mol for the first and second water molecules, respectively. They agree within error limits with the ones predicted by ab initio calculations done at the CCSD(T)/aug-cc-pVQZ//CCSD/aug-cc-pVDZ level of theory. We show that the multiple-well MEM approach described here yields superior results in systems with several low-lying minima, which is the typical situation for hydrated ions.
Related collections
Most cited references47
- Record: found
- Abstract: not found
- Article: not found
Algorithm 448: number of multiply-restricted partitions
- Record: found
- Abstract: not found
- Article: not found
Statistical modeling of collision-induced dissociation thresholds
- Record: found
- Abstract: not found
- Article: not found