- Record: found
- Abstract: found
- Article: not found
FRET Reveals the Formation and Exchange Dynamics of Protein-Containing Complex Coacervate Core Micelles

Read this article at
Abstract
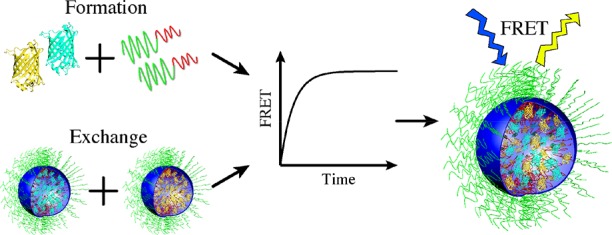
The encapsulation of proteins into complex coacervate core micelles (C3Ms) is of potential interest for a wide range of applications. To address the stability and dynamic properties of these polyelectrolyte complexes, combinations of cyan, yellow, and blue fluorescent proteins were encapsulated with cationic-neutral diblock copolymer poly(2-methyl-vinyl-pyridinium) 128- b-poly(ethylene-oxide) 477. Förster resonance energy transfer (FRET) allowed us to determine the kinetics of C3M formation and of protein exchange between C3Ms. Both processes follow first-order kinetics with relaxation times of ±100 s at low ionic strength ( I = 2.5 mM). Stability studies revealed that 50% of FRET was lost at I = 20 mM, pointing to the disintegration of the C3Ms. On the basis of experimental and theoretical considerations, we propose that C3Ms relax to their final state by association and dissociation of near-neutral soluble protein–polymer complexes. To obtain protein-containing C3Ms suitable for applications, it is necessary to improve the rigidity and salt stability of these complexes.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa Predictions.
- Record: found
- Abstract: not found
- Article: not found
A constrained regularization method for inverting data represented by linear algebraic or integral equations
- Record: found
- Abstract: found
- Article: not found
