- Record: found
- Abstract: found
- Article: found
Ustekinumab in the Treatment of Generalized Pustular Psoriasis in a Pregnant Patient
Read this article at
Abstract
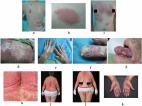
Although the use of biologics has led to great improvement in psoriasis patients, the treatment of psoriasis during pregnancy still faces many challenges. We herein report on a 29-year-old pregnant woman treated with ustekinumab for generalized pustular psoriasis. Upon becoming pregnant, the woman underwent continued treatment with ustekinumab in the first trimester. We also considered the need for neonatal vaccination. The patient discontinued ustekinumab therapy in the second trimester, and during the period of drug discontinuation we noted a slight rash recurrence. The patient was treated with ultraviolet B phototherapy and topical corticosteroids, and the rash was localized to the abdomen. However, in the 27th week of pregnancy, the patient was infected with COVID-19, which made the condition worse. The rash erupted rapidly and spread throughout her body, and she experienced a high fever with her blood count showing augmented numbers of white blood cells. The patients self-administered 0.3 g of acetaminophen three times per day, and after four days her core body temperature was 38.0°C; the rash, however, did not diminish. We diagnosed an outbreak of generalized pustular psoriasis and treated the patient with ustekinumab. The rash resolved quickly, and a healthy newborn was delivered by caesarean section at 39 weeks.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
CME Part I Psoriasis: Which Therapy for Which Patient Psoriasis comorbidities and preferred systemic agents
- Record: found
- Abstract: found
- Article: found
Clinical Course and Characteristics of Generalized Pustular Psoriasis
- Record: found
- Abstract: found
- Article: found